Abstract
Aims: Limited data are available on the changes that occur at the dilated site many years after coronary balloon angioplasty. The development of bioabsorbable stents may increase the importance of understanding the long term changes that occur in an unscaffolded coronary artery following balloon-mediated injury.
Methods and results: This study evaluated, by serial quantitative angiography, the natural history of changes that occurred in the dilated segment between early (mean seven months), late (mean 4.5 years) and very late (mean 17 years) follow-up after balloon angioplasty.
Of 127 consecutive patients (174 lesions) with successful coronary angioplasty, 125 underwent early, 84 late and 47 very late angiographic follow-up (75% of eligible survivors). The mean lesion diameter stenosis decreased from 36±11% at early to 26±15% at late follow-up (p<0.0001), and then increased again to 35±25% by very late follow-up (p=0.003). Although stenosis severity at early follow-up angiography predicted lesion regression at late follow-up, there was no significant correlation between late and very late follow-up lesion severity.
Conclusions: After coronary angioplasty, lesion regression at the dilated site from 7 months to 4.5 years is followed by slow lesion progression over the next 12 years.
Introduction
Many clinical and angiographic studies have assessed the response to vessel injury following coronary balloon angioplasty. Neointimal proliferation and negative remodelling commonly lead to restenosis within the first six months following the procedure1-10; beyond this time restenosis is uncommon. We11 and others12,13 have demonstrated that lesion regression occurs over the subsequent 3-5 years.
Stents eliminate elastic recoil and scaffold the artery during the repair process following balloon-mediated vessel injury. Drug-eluting stents reduce restenosis through a reduction in neo-intimal proliferation, but at a cost of delayed re-endothelialisation and increased late stent thrombosis14. Unlike balloon angioplasty alone, stent deployment may be associated with late luminal narrowing beyond four years15. Recently developed bio-absorbable stents may confer the early scaffolding benefit of stents, whilst allowing the artery to behave more like an angioplastied vessel in the long term16,17. The long-term natural history of balloon angioplasty-treated lesions may once again be of clinical relevance.
This observational study followed a cohort of consecutive patients undergoing balloon angioplasty who agreed to clinical and angiographic follow-up. We extend our previous findings11 by describing the changes that occur between late (mean 4.5 years) and very late (mean 17 years) follow-up after intervention.
Methods
Study population
Patients undergoing coronary balloon angioplasty during the initial experience at Green Lane Hospital, Auckland, New Zealand between 1981 and 1986, routinely underwent follow-up angiography approximately six months following the index procedure. A prospective follow-up protocol that included late (mean 4.5 years) repeat angiography was devised to assess the long-term results of the procedure. The study was subsequently extended to assess very late (mean 17 years) changes after the index procedure.
Patients who had successfully undergone angioplasty to de novo native coronary artery stenoses and who had undergone both early and late follow-up angiography (n=84) were eligible for very late angiographic follow-up if they had remained free from death or target vessel revascularisation by either repeat percutaneous coronary intervention or coronary artery bypass surgery. If patients had undergone a clinically-indicated angiogram >10 years after their index angioplasty procedure, this angiogram was used for the very late follow-up study. Of the 54 study eligible patients, 47 patients with early, late and very late angiographic follow-up constituted the current study population (Table 1).
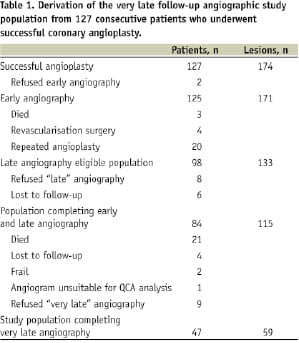
Of this group, 12 patients underwent a clinically-driven and 35 a protocol-mandated, very late coronary angiogram. The study protocol was approved by our local ethics committee and written informed consent was obtained from all patients.
Angioplasty procedure, follow-up angiography and quantitative angiographic analysis
The angioplasty procedure, follow-up angiography and quantitative angiographic analysis have previously been described11. In brief, all coronary angioplasty was performed via the femoral arterial route. A 10,000 IU bolus of unfractionated heparin was administered at the start of the procedure and additional boluses of 5000 IU administered hourly, for longer procedures. In the early patients angioplasty was performed employing 9 Fr guiding catheters and fixed-wire angioplasty balloons. Later patients had procedures performed with over-the-wire balloons via 8 Fr guiding catheters. Procedural success was defined as <50% residual coronary diameter stenosis and freedom from in-hospital myocardial infarction, surgical revascularisation or death.
Five coronary angiograms were recorded in each study patient: before and immediately after angioplasty, and at early, late and very-late follow-up. For the follow-up angiograms, sublingual nitroglycerin was routinely administered before image acquisition. Orthogonal projections that best demonstrated the stenosed segment were identified before angioplasty. At follow-up, the same projections were chosen and care was taken to reproduce the field size, rotation, angulation, image-intensifier height and table height.
Quantitative coronary angiographic measurements were made using the Cardiovascular Measurement Medical Imaging Systems (CMS-MEDIS). Calibration was performed either with a 1 cm calibration grid filmed at the isocentre of each projection at each image-intensifier height, or from a non-tapering portion of the angiographic catheter. For any individual patient, the same method of calibration was used for each of the five angiograms (grid or catheter). The maximum percent diameter stenosis (DS), lesion minimum luminal diameter (MLD) and automatically determined reference vessel diameter (RVD) were calculated. The analysis was performed by a single technician trained in the use of the CMS system.
We have previously shown11 that the accuracy (mean difference between the repeated measurements) for both MLD and RVD measurements by this method was 0.01 mm. The precision (standard deviation [SD] of these differences) for both was 0.17 mm. For percent DS, accuracy was –0.53% and precision was 7.28%.
Statistical analysis
Continuous variables are expressed as mean (SD) or as frequencies. Repeated measures analysis of variance was used to establish any significant differences over time in percent DS, lesion MLD and RVD in all lesions. Comparisons between groups were made with the Bonferroni modification for multiple testing (SAS statistics package). For other continuous variables, comparisons between times were carried out using a paired t test with the Bonferroni modification. Categorical variables were compared using Fisher’s exact test.
Results
The derivation of the study population is depicted in Table 1. Of the 84 patients who had both early (mean seven months, range 1-13 months) and late (mean 4.5 years, range 1.5 to 7.8 years) coronary angiography after successful angioplasty, 21 died prior to their planned very late angiographic follow-up (12 cardiac deaths, six respiratory deaths, two malignancies, one abdominal aortic aneurysm rupture). This left a potential study population of 63 patients, of whom 54 returned for clinical follow-up and 47 (75%) underwent further very late follow-up angiography, at a mean of 17 years (range 11 to 21.5 years) after their angioplasty procedure. Of the 16 study-eligible patients who did not undergo very late follow-up angiography, nine declined the procedure, one had angiography performed elsewhere in a format unsuitable for quantitative angiographic analysis, two were considered too frail to undergo angiography, and four were lost to follow-up.
The clinical characteristics at baseline and at very late follow-up of the study cohort are depicted in Table 2.
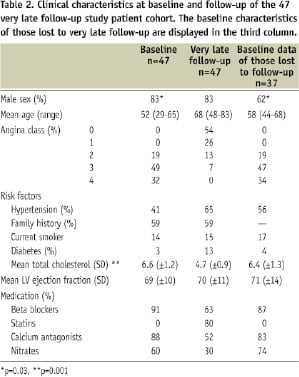
There was no significant difference in the occurrence of cardiac risk factors between those patients in whom the lesion had progressed, compared to those in whom the lesion had regressed. There was a significant reduction in serum cholesterol from baseline to very late follow-up consistent with the increased use of lipid-lowering medications (p≤0.001). Table 2 also shows the clinical characteristics of those patients lost between late and very late follow-up. Apart from being more likely to be female (p=0.03), there were no significant differences between these patients and the study population. Of the 47 patients in the very late follow-up angiography group, 20 patients had undergone further PCI/PTCA of new de novo lesions and 4 CABG, which did not subtend the (patent) target segment. There was no significant difference in further intervention rates when compared to those who had died.
The mean MLD decreased from 2.13±0.54 mm at late to 1.80±0.72 mm (p<0.001) at very late angiographic follow-up, and a corresponding increase in mean DS from 26±15% at late to 35±25% (p=0.003) at very late follow-up (Figure 1).
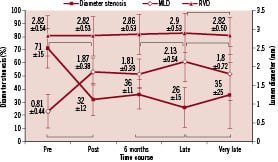
Figure 1. Serial changes in reference vessel diameter (RVD), lesion minimal luminal diameter (MLD) and percent diameter stenosis (DS), before angioplasty, immediately after angioplasty, at early follow-up, late follow-up and very late follow-up for the 59 lesions in the study population of 47 patients. Values are mean (SD). P<.005 for comparison between the points linked by brackets.
The RVD also reduced significantly between late and very late follow-up, from 2.90±0.53 mm to 2.82±0.50 mm (p=0.0017). The very late MLD and DS were very similar to those at six months.
Because stenosis severity at early angiography predicted the extent of stenosis reduction at late angiography11, we divided lesions into three groups according to stenosis severity at early angiography (Figure 2).

Figure 2. Change in diameter stenosis over time divided into tertiles based upon stenosis severity at early follow-up angiography. The greater the early diameter stenosis, the greater the degree of lesion regression at late follow-up. Between late and very late follow up, lesions tended to progress.
In those with lesions <30% diameter stenosis at early follow-up, there was no significant change in stenosis severity at late or very late follow-up. In the remaining two groups (stenosis severity 30-39% and ≥40% at early angiography), a significant reduction in stenosis severity at late angiography was followed by a non-significant trend to lesion progression by very late follow-up.
Twice the standard deviation of repeated measurements in the intra-observer study was 0.34 mm. Of the 59 lesions that had both late and very late angiographic follow-up, 20 lesions (34%) progressed by >0.34 mm and 5 (8%) regressed by >0.34 mm. One complete vessel occlusion that had occurred by late follow-up was excluded from this analysis.
Discussion
This study reports the longest clinical and angiographic follow-up undertaken on a cohort of consecutive patients having coronary balloon angioplasty for de novo coronary artery stenoses. A unique feature is the four serial angiograms performed in each patient over 17 years.
Restenosis at balloon-dilated sites typically occurs within the first six months. A rigorous evaluation using serial quantitative angiography at 1, 3, 6 and 12 months demonstrated that most restenosis occurs between one and three months after angioplasty, with little change in minimum luminal diameter from 6 to 12 months1. We previously demonstrated that lesion regression frequently occurs between seven months and 4.5 years after angioplasty11, a finding confirmed by several other studies3,12,13,18. Beyond this time frame, our finding of slow progression at the site of most dilated lesions is consistent with other studies of shorter follow-up12,13. There appears therefore to be a continuum of changes occurring after coronary angioplasty that can now be extended to four distinct phases; phase I occurring in the first six months, characterised by restenosis; phase II, from 6-12 months, a period of plaque stabilisation with little change in minimum luminal diameter; phase III, from 12 months to five years, characterised by lesion regression; and now phase IV, beyond five years, characterised by slow lesion progression.
Restenosis after coronary angioplasty is multifactorial. Apart from early elastic recoil19, balloon-mediated vessel injury promotes neointimal proliferation of smooth muscle cells and production of extracellular matrix20-23. Pathologic and intravascular ultrasound studies have shown that negative remodelling or vessel shrinkage is also an important contributor to the restenotic process18,24-26. Animal27 and human autopsy22 studies have found that neointimal proliferation after balloon-mediated vessel injury reaches a maximum before six months and then regresses. A reduction in extracellular matrix and fibrotic scar formation associated with maturation of smooth muscle cells may contribute to late regression after angioplasty15,22,27. Shear stress contributes to plaque growth and vascular remodelling25. Low shear stress promotes plaque growth with outward remodelling, regions of physiological shear stress remain quiescent, and sites of increased shear stress exhibit outward remodelling without plaque growth28-30. Early restenosis after angioplasty may lead to compensatory outward remodelling via increased shear stress until shear stress is restored to baseline values13.
The very late changes we observed may be due to infiltration by lipid-laden macrophages in the shoulder regions of the sub-endothelial space, as found in a pathological study of angioplasty lesions studied seven or more years later31. After more than 10 years, thin fibrous caps heavily infiltrated by foam-cells were seen around the circumference of the lumen at balloon-dilated sites. These changes suggest that very late progression is a progressive atherosclerotic process.
In contrast to the late luminal response after angioplasty, there is limited data on late changes following stenting. Kimura and colleagues32 demonstrated that, after implantation of Palmaz-Schatz stents, there was no change in MLD between six months and one year, and late luminal enlargement between one and three years after stent deployment. However, further angiographic follow-up demonstrated that late luminal re-narrowing was common beyond four years, perhaps due to chronic inflammation occurring around the stent struts15.
The time course of in-stent proliferation may be different following drug-eluting stent deployment. Four year angiographic and intravascular ultrasound (IVUS) follow-up of patients following implantation of a sirolimus-eluting stent demonstrated no significant change in in-stent lumen dimensions over this time period33. Similar findings were reported in a diabetic population with 18 month angiographic and intravascular ultrasound follow-up34. However, Li et al35 recently reported a comparison between early (≤ 270 days) and late (≥ 270 days) angiographic follow-up in a cohort of 333 patients undergoing sirolimus-eluting stent implantation. In-segment restenosis was greater in the late compared with the early group (16.7% vs 7.9%, p=0.013) suggesting that there may be “late catch-up” due to neointimal growth beyond nine months. A subset of patients enrolled in TAXUS II trial underwent QCA and intravascular ultrasound at six months and two years. Unlike those who received bare metal stents, neointimal volume by ultrasound increased between six months and two years in those with a paclitaxel-eluting stent, an interesting finding discordant with the QCA measurements36. In the recent SPIRIT II study, there appeared to be attenuation of the advantage of everolimus-eluting over paclitaxel-eluting stents in reducing late lumen loss, between six months and two years37.
Early studies of bioabsorbable stents show promise16,17. They may combine the early benefits of stents (eliminating elastic recoil, tacking up dissections, reducing restenosis through local anti-proliferative drug delivery), whilst avoiding some of the later disadvantages (impairment of restoration of vasomotion, limiting future percutaneous or surgical intervention, hypersensitivity reactions, aneurysm formation, late stent malapposition, late stent thrombosis)38. The natural history of balloon-dilated lesions may give us some insight into how lesions temporarily scaffolded by bioabsorbable stents will behave over the long term.
Study limitations
The major limitation is the attrition of the study population over the long duration of the study. Many of the initial cohort had died or were very frail and elderly by 17 years of follow-up. A number of patients were lost to follow-up due to cardiac death. In some cases this may have been due to progression of disease at the previously treated site. Although there appeared to be few differences between the study population and the remainder, a “survivor bias” is also possible.
A further limitation relates to the use of statins, which were clinically introduced in the period between late and very-late follow-up. The regression reported at late follow-up was seen before the introduction of statins, but later progression was seen when 80% of patients were taking a statin, suggesting that even more progression may have been observed had statins not been introduced.
Although this was a prospective study, patients who underwent a clinically driven angiogram ≥10 years following their index angioplasty were included in the very late angiographic follow-up cohort. Hence the interval between late and very late follow-up angiography ranged from 11 to 21 years.
Conclusion
After coronary angioplasty, lesion regression at the dilated site occurs between seven months and 4.5 years. Beyond this time, there appears to be slow lesion progression over the next 12 years, such that lesion severity at seven months and 17 years is very similar.
Acknowledgements
The first phase of this study was initiated by Dr. Tony Roche, Director of Interventional Cardiology at Green Lane Hospital until his untimely death in 1993. We thank Irene Zeng for performing the statistical analyses and catheterisation laboratory staff for their expert assistance in acquiring the angiographic data. We are particularly grateful to all the patients who agreed to participate in this long-term study.

