Abstract
Aims: To describe the impact of a steerable device on procedural success and times. The Venture™ wire control catheter (VWC-St. Jude Medical, Maple Grove, MN, USA), facilitates wire orientation providing excellent backup support and may therefore assist in cases in which conventional approaches have failed.
Methods and results: We describe all cases in which the VWC catheter was used at our institutions. The device was employed after prolonged attempts with standard wires had failed. We analysed procedural success and complication rates, as well as the impact of this device on procedural times. We evaluated 18 cases. The mean time from the first wire attempt to the end of the procedure was 58 minutes. Lesion crossing attempts with standard wires were performed for a mean time of 23 minutes (range 10-45 minutes). The VWC catheter was employed as the last resort, leading to procedural success in 14 cases. Lesion crossing with the VWC catheter was achieved in a mean time of four minutes (range one to 15 minutes). There were no device-related complications.
Conclusions: Our experience shows how the VWC catheter can turn procedural failures into successes, significantly impacting procedural times in different challenging scenarios. This device may represent an extremely useful addition to the interventionalist’s armamentarium.
Introduction
The continuous progress in guidewire, balloon and stent technology, as well as the growing operator experience have significantly increased the procedural success rate of percutaneous coronary interventions (PCI). Despite this, certain anatomical conditions, such as severe vessel tortuosity or angulation, stent jailed side-branches and chronic total occlusions (CTO) continue to represent a challenge for the interventionalist, by making target vessel and lesion access difficult1. Drug-eluting stents have markedly reduced restenosis rates and hence encouraged the treatment of far more complex coronary lesions. This has amplified the importance of lesion negotiation2. Currently, complex lesion PCI is associated with lower success rates, increased complications3-4 and higher costs, as additional materials are often needed to tackle these anatomical subsets5.
Novel deflectable-tip steerable devices may help in directing guidewires, providing good back-up, and assist in challenging cases in which attempts with standard techniques have failed. Furthermore, they may offer additional benefits by reducing procedural times, radiation exposure, contrast volume, and costs.
We report here our clinical experience with the use of the Venture™ Wire Control (VWC) catheter (St. Jude Medical, Maple Grove, MN, USA), employed in coronary vessels and lesions with unfavourable anatomic features after failure of conventional approaches.
Methods
Study population
We described all cases in which the VWC catheter was used in our two institutions between February 2005 and March 2007. The device was employed as the last resort after prolonged attempts using state-of-the-art guidewires had failed, due to inability to access the target vessel or to cross the target lesion. All patients provided informed consent for both the procedure and subsequent data collection and analysis for research purposes. Procedural anti-platelet therapy and heparin dosing followed standard protocols6.
Study endpoints
We analysed device procedural success and complication rates, as well as the impact of this device on procedural times.
Definitions
Device success was defined as guidewire placement in the vessel true lumen at least 10 mm distal to the target lesion. Angiographic success was defined as a final angiographic residual coronary stenosis less than 20% by visual estimation. Procedural success was defined as device and angiographic success in the absence of any procedural complication. Myocardial infarction (MI) was defined according to the joint ESC/ACC consensus document7: two times increase the upper limit of normal for total CK in conjunction with elevation in the CK-MB levels. In all patients, CK and CK-MB were evaluated every six hours, three times following the procedure, and until normalisation if they were elevated.
Device and procedural characteristics
The VWC catheter and instruction for its use have been previously described in detail8. This device is a 6 Fr compatible over the wire or monorail catheter with a soft deflectable radio-opaque tip (Figure 1).
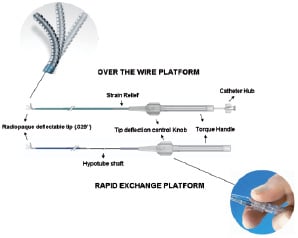
Figure 1. The Venture™ wire control catheter. Over-the-wire and rapid exchange platforms.
The catheter inner lumen accommodates any 0.014”-inch guidewire. Clockwise turning of a rotating knob, located on the proximal handle, deflects the catheter tip up to 90°, precisely directing the guidewire and providing back-up support. The key catheter specifications are summarised in Table 1.

In our series, the VWC catheter was either introduced as a single unit after loading a standard guidewire of choice into the lumen, or it was advanced into the guide catheter over a previously placed exchange-length guidewire, which had been placed proximal to the target lesion. In all cases, the VWC catheter was advanced in its straight configuration over the guidewire. The distal radio-opaque tip of the catheter was deflected to the desired angle to allow guidewire advancement to the target vessel/lesion and to provide simultaneous support for lesion crossing. Following successful lesion negotiation, the VWC catheter was returned to its straight configuration and removed, and the PCI was performed with standard techniques.
Statistical analysis
Continuous variables are presented as means±standard deviation and categorical variables as frequencies (%). The normality of the distribution of the continuous variables was tested using the Kolmogorov-Smirnov test. We compared the time expended in lesion crossing prior and after the VWC was employed with the paired Student’s T test. A p-value of <0.05 was considered to be statistically significant and reported p-value is two-sided. Statistical analysis was performed using SPSS 11.5 (SPSS Inc., Chicago, IL, USA).
Results
During the study period 5,090 PCIs were performed at our two institutions. The VWC catheter was evaluated in 18 patients in whom prolonged attempts with standard wires had failed. The demographic and clinical characteristics of these patients are summarised in Table 2.
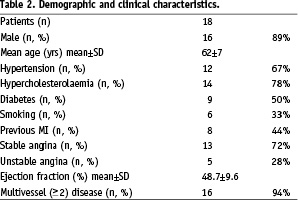
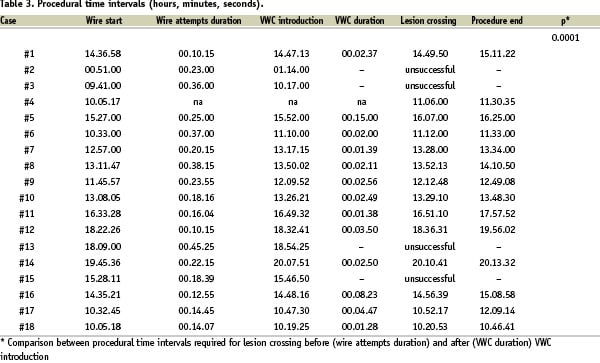
The mean time from the first wire attempt to the end of the procedure was 58 minutes (Table 3).
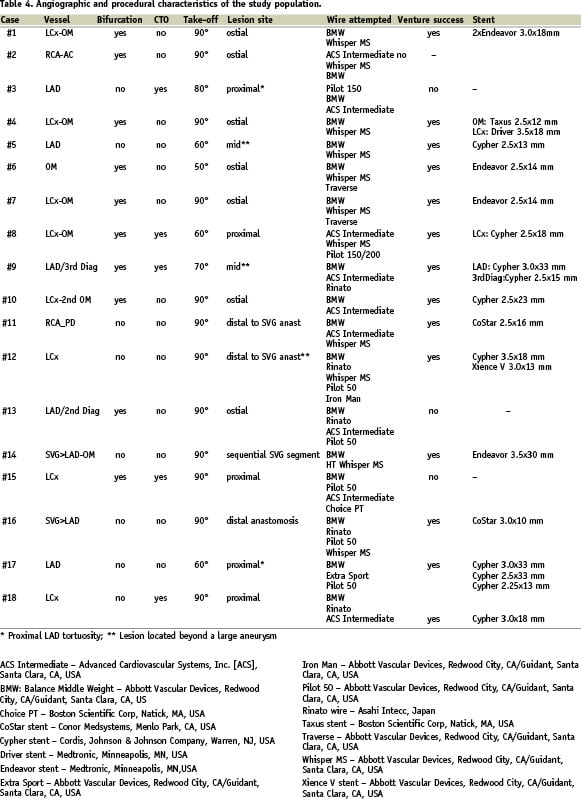
At least two different wire types were attempted before the VWC was introduced (Table 4).
In all the cases of CTO, wire manoeuvring with the use of a 1.5 mm over the wire balloon (Maverick, Boston Scientific Corp, Natick, MA, USA) was considered the basic approach, prior to switch to the use of the VWC catheter.
The time when the VWC was introduced was recorded in all, but one case. Lesion crossing attempts with standard wires prior to VWC introduction were performed for a minimum of 10 to a maximum of 45 minutes, with an average time of 23. Lesion crossing once the VWC was employed was achieved in a minimum of one to a maximum of 15 minutes, with a mean time to cross the lesion of four minutes. In particular, in two cases in which unsuccessful attempts with standard wires had been performed for 37 and 38 minutes, respectively, lesion crossing was achieved within two minutes after the VWC was employed (Table 3).
Both device and angiographic successes were achieved in 14 cases (78%). There were no device related angiographic complications, specifically no cases of coronary dissection or perforation. In three cases (two unsuccessful CTO recanalisations and one very angulated and tortuous sub-occluded side branch), the wire entered the false lumen. In only one of these was there a residual guidewire mediated dissection at the end of the procedure. One periprocedural MI occurred leading to a procedural success rate of 72%. No other events occurred either in-hospital or within 30 days.
The device was employed in (Table 4):
– Seven bifurcation lesions
– Five CTOs
– Two tight stenoses located distal to a large coronary aneurysm
– Three lesions involving saphenous vein grafts (SVG)
– One severely calcified and tortuous native coronary artery.
Seven bifurcation lesions required the employment of the VWC after conventional techniques failed, either due to angulated side branch take-off (> 80 degrees) or tortuous and very diseased side branches. Bifurcation lesions were defined according to the SYNTAX classification9 and were as follows: four type E lesions, two type B lesions, and one type F lesion. In one case the side branch originated with an extremely angulated take off, in another it was sub-occluded at the ostium and tortuous and severely diseased in the proximal segment. In three cases the side branch had both a very angulated take off and was sub-occluded at the ostium (Figure 2); in the other two cases the side branch became occluded and sub-occluded, respectively, after stent implantation in the main branch (see below).
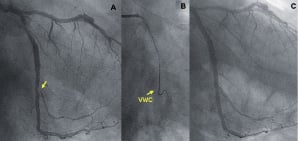
Figure 2. An angulated bifurcation lesion. Right anterior oblique (RAO) caudal view showing an obtuse marginal (OM) branch sub-occluded at the ostium and arising from the left circumflex artery (LCx) with a very angulated take off (A, arrow), the VWC (arrow) allowing successful lesion crossing (B) and final angiographic result (C).
Success after VWC employment was achieved in all these lesions except for the two stent-jailed side branches. In all the successful cases bifurcation lesions were treated with provisional stenting technique, except for one case where the occurrence of plaque shift into the side branch, after stenting of the main, required the implantation of an additional stent in the side branch, performed using the reverse crush technique10.
In all five cases of CTOs the stump morphology was blunt (Figure 3).
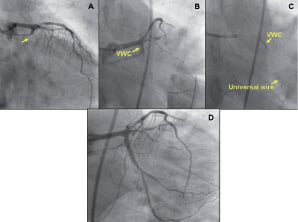
Figure 3. A blunt CTO. RAO cranial (A) and antero-posterior (AP) caudal (B) views showing a blunt chronic total occlusion (CTO) located in the proximal LCx arising with a 90° take off from the left main (LM). By precisely directing the guidewire tip (B, insert) towards the LCx, simultaneously providing enhanced support, the WVC allowed lesion crossing (C). AP caudal view showing the final result (D).
Contralateral collaterals were present in all and bridging collaterals in three cases. In one case, the CTO was located in the mid-portion of a tortuous left anterior descending artery (LAD), originating from the left main with an angulated take off. In this case, lesion access was not possible even after the VWC was employed. In four cases the VWC was used to access the occluded main vessel at the level of a branching point with a side branch arising just before or from the occluded segment. In one of these two cases, the access to a CTO lesion located in the mid portion of the LAD was made even more challenging by the presence of a large aneurysm in the proximal LAD (Figure 4).
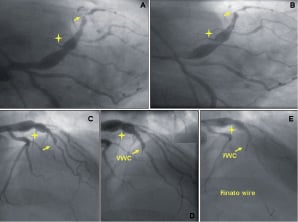
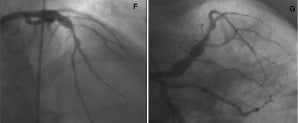
Figure 4. A large coronary aneurysm followed by a blunt CTO. AP caudal (A, B) and RAO cranial (C-E) views showing the presence of a large aneurysm (marker) in the proximal left anterior descending artery (LAD) and a blunt CTO of the mid LAD at the bifurcation with a third diagonal branch, which has in turn a critical ostial stenosis. Attempts performed with standard guidewires were unsuccessful due to the wire prolapsing into the main artery immediately after a steering force was applied in order to engage the lesion (B). The VWC (D, arrow) due to its deflectable tip (D, insert) was of crucial importance in orientating the wire to cross the CTO lesion (E). RAO cranial (F) and AP caudal (G) views showing the final result.
The VWC was crucial in allowing crossing of this lesion and therefore its treatment, which was performed with a step crush technique10.
In two other cases, lesion access with standard techniques was prevented by the presence of a large coronary aneurysm in the segment proximal to the target lesion. In one of these cases the aneurysm was located in the mid LAD. In the other, the aneurysm was located in the native left circumflex artery (LCx) distal to a very angulated SVG anastomosis (Figure 5).
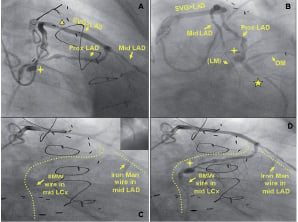
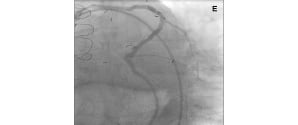
Figure 5. An angulated SVG anastomosis and coronary aneurysm. RAO cranial (A, C, D) and Left Anterior Oblique (LAO) caudal (B) views showing a saphenous vein graft (SVG) to mid LAD. There is a focal critical stenosis in the proximal segment of the SVG body (A, triangle). The proximal LAD, as well as the LCx, originating from a poorly visualised LM are filled by retrograde flow. There is an aneurysm in the proximal LCx (A, B, D: marker), just before the origin of an OM branch (B). The target lesion is located in the mid portion of the LCx (B, star). The VWC catheter allowed us to wire the native LAD retrogradely via the angulated SVG anastomosis (C, insert) and to steer the wire inside the coronary aneurysm (C, D). AP caudal (E) view showing the final result.
The VWC catheter proved useful not only to wire the native vessel through the SVG anastomosis, which was extremely difficult due to the angulated take off, but also to steer the wire inside the coronary aneurysm in order to reach and cross the lesion.
The VWC was employed in three other lesions involving angulated SVGs. In one, the target vessel was a diffusely diseased posterior descending artery (RCA-PD), which was sub-occluded just distally to a very angulated (>90 degrees) distal SVG anastomosis (Figure 6).
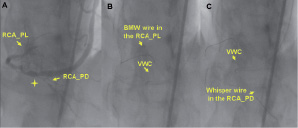
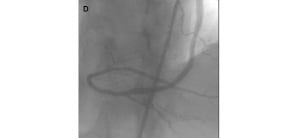
Figure 6. An angulated retroflexed SVG anastomosis. LAO cranial view showing the distal portion of a sequential SVG anastomosed to an OM and to the posterior descending of the right coronary artery (RCA-PD). The postero-lateral branch (RCA-PL) is visualised by retrograde flow. The target lesion is the RCA-PD, which is sub-occluded just beyond the distal SVG anastomosis (A, marker). The VWC precisely oriented the wire tip towards the RCA-PD distal to the angulated retroflexed SVG anastomosis (B), allowing lesion crossing (C) and treatment (D).
In another case, the VWC provided access to a severely diseased, tortuous and angulated distal portion of a sequential SVG to LAD/ obtuse marginal (OM). In the third case the VWC was of critical importance to gain retrograde access to a native LAD occluded just proximal to the distal SVG anastomosis (Figure 7).
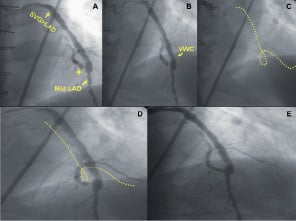
Figure 7. Retrograde access to native vessel CTO via an angulated SVG anastomosis. RAO cranial view showing the distal anastomosis of a SVG to the mid LAD. The native vessel is occluded just proximal to the distal SVG anastomosis (A, marker). The VWC tip, precisely directing the wire to the lesion site (B), allowed retrograde access to the native vessel (C, D) and its treatment (E).
Finally, the VWC proved its utility in a case of diffusely calcified and severely tortuous LAD, where negotiation of a proximal lesion with standard techniques was not possible despite prolonged attempts.
Device success was not achieved in four cases. Two were blunt stump CTOs, located in an angulated and tortuous mid LAD and in the proximal portion of a LCx, respectively. Though an apparently correct orientation was provided by the VWC, the wire always entered the false lumen and the operator decided to terminate the procedure.
The third case involved a large acute marginal branch (≈2.5 mm diameter), which became completely occluded, after recanalisation and stenting of an occluded proximal RCA. Wiring of the stent jailed vessel was not possible even with the use of the VWC. The occlusion of this branch caused a periprocedural MI. The remaining unsuccessful case involved a small (<2.25 mm), second diagonal branch which became sub-occluded at the ostium after mid LAD stenting was performed. The wire was precisely directed by the VWC, but a dissection which had occurred during previous attempts with wires alone prevented true lumen negotiation. However this dissection was not flow-limiting and no periprocedural MI occurred (Figure 8).
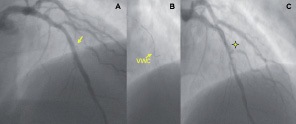
Figure 8. A stent jailed side branch. RAO cranial view showing sub-occlusion at the ostium of a small 2nd Diagonal branch (arrow) occurring after mid LAD stenting (A), the VWC (arrow) directing the tip of the wire towards the Diagonal origin, (B) and the presence of a non flow-limiting dissection (marker) preventing true lumen negotiation (C).
Discussion
We described our experience with the VWC catheter in 18 patients where standard state-of-the-art techniques failed. To our knowledge this is the first case series of consecutive patients in which this device was used.
The main finding of this study is that this device appears safe and effective, not only leading to an angiographic success rate of 78%, in procedures otherwise unsuccessful, but also doing it in a timely fashion. The time saving in lesion crossing was indeed remarkable, as compared with previous attempts performed with conventional techniques. In addition, the VWC catheter turned procedural failures into successes in a variety of different kind of lesions, highlighting its versatility and usefulness in different complex settings.
Dealing with coronary scenarios similar to those approached in our study, with tortuous and complex anatomy, extreme angulation of side-branch ostia, or lack of control at the interface of CTO is not uncommon1-2. To tackle these challenging situations the interventionalist may utilise a variety of techniques. A combination of over-the-wire balloons or different guiding catheters may enhance back-up, the use of a microcatheter being essential especially for CTO lesions, while a variety of guidewires reshaped with different bends according to the vessel anatomy may be tried in an attempt to improve steerability11. Gershony et al12 have shown that the use of an inflated perfusion balloon just distal to an acute origin of a branch vessel may be useful to allow the deflection of a guidewire into the branch vessel. Despite these approaches, torquability may be diminished in the presence of large coronary aneurysms, extreme tortuosity or angulation, making manoeuvring difficult and lesion attainment and crossing often unsuccessful. In these settings the use of the VWC catheter may be of particular advantage. The tip of the VWC catheter can be deflected to orientate and direct the guidewire up to 90° by rotation of the control knob located on the proximal handle. This feature, associated with rotation of the entire catheter shaft, allows steering the guidewire tip in all planes. This innovative mechanism enhances guidewire deliverability through challenging lesions, simultaneously providing excellent back-up support and increased directional pushability.
Initial data on the use of the VWC were provided by McClure et al8 who described three patients with challenging coronary anatomy successfully treated without any device-related complication. These authors provided descriptive data, without delineating whether the use of the VWC was preceded by other techniques. Only three other cases in which the use of the VWC was required after prior attempts with standard guidewires were unsuccessful have been reported13-14.
As shown in our experience, one promising area for VWC catheter may be the percutaneous treatment of CTO. Despite a CTO being found in about 30% of all coronary diagnostic procedures, an attempted revascularisation of these lesions accounts for less than 8% of all PCI15. This could be, at least in part, related to the challenge offered by these lesions, with success rates ranging between 70% and 80%16-18. Indeed, inability to cross the occlusion with the guidewire is the most common obstacle, accounting for more than 60% of procedural failures19,20. In addition, CTO located at branching points do not allow the operator to advance directly into the lesion and the wire often prolapses into adjacent branch vessels16,21. By precisely directing the guidewire tip towards the desired lesion point and increasing pushability due to the enhanced support, the VWC catheter may play an indispensable role in improving the success rate of CTO interventions, in particular when dealing with flush CTOs with blunt stump. In addition, the employment of this device may potentially reduce the risk of vessel dissection and perforation, resulting in safer procedures.
We have also seen the utility of the VWC catheter in crossing coronary stenoses located at the ostium of vessels with an angulated origin. In these cases we were able to deflect the catheter tip and advance the guidewire beyond the target lesion and perform PCI successfully. In such situations lesion crossing accounts for almost all the procedural difficulty.
Albeit a rare situation, lesions located beyond coronary aneurysms may be very challenging particularly with large aneurysms and when the aneurysm afferent and efferent coronary segments are asymmetrically located. In the cases of this type reported in our series, the use of the VWC catheter allowed us to steer the wire inside the aneurysm and enhanced the directional pushability that was needed to reach and cross the distal target lesion. In one of these case, involving a CTO following a large aneurysm, the VWC had a double role, both orientating the wire through the aneurysm, allowing the reaching of the lesion, and further orientating and supporting the wire across the coronary occlusion.
Finally, lesions located in native vessels beyond angulated distal SVG anastomoses or along severely tortuous vessels may be difficult to reach and cross, and the VWC catheter may offer an additional option to successfully deal with these lesions.
Is to be noted that the first two cases in which device success was not achieved were performed in a very early phase of our experience, when the operators were not confident with this device. Improvement in the learning curve with the VWC may further increase the associated success rates.
Theoretical limitations of the VWC catheter may include difficulties in tip deflection and catheter shaft rotation in small calibre vessels and the potential for vessel injury during catheter manipulation. However, it is reassuring that we did not observe any device-related complication, neither in vessel of 2.25-2.5 mm diameter.
In conclusion, our experience demonstrates that, although not a device used in routine clinical practice, the VWC catheter plays an important role in certain coronary subsets in which conventional guidewires have failed, particularly when anatomical features make vessel access and lesion crossing challenging. This device may further improve procedural success rate in the current PCI era of ever increasing lesion complexity.

