Abstract
Aim: To report clinical and angiographic follow-up at 6 months after implantation of the Tryton Side-Branch Stent™ dedicated to bifurcation, in conjunction with standard workhorse drug-eluting stent (DES).
Methods and results: Patients having angina or silent myocardial ischaemia with de novo bifurcation lesions were enrolled at three sites. The Tryton™ stent was first placed in the side-branch and a standard DES subsequently implanted in the main-branch. The primary endpoint was freedom from in-hospital major adverse cardiac events (MACE) following procedural success. Angiographic and clinical follow-up was performed at 6-months. Quantitative coronary angiography (QCA) was analysed with a new dedicated bifurcation software. Thirty patients were enrolled. At six months clinical follow-up was available in 100% with angiographic follow-up performed in 78%. The primary endpoint was met in 93.3%. The MACE rate at 6 months was 9.9%. QCA analysis demonstrated late luminal loss of 0.17 mm with no restenosis in the side branch.
Conclusions: In conjunction with a workhorse DES to treat bifurcation lesions, the Tryton™ side-branch stent demonstrated acceptable clinical outcome and low side-branch late loss at six months.
Introduction
Bifurcations of coronary arteries are predilection sites for atherosclerosis and expansive remodelling1. Percutaneous coronary intervention typically involves a bifurcation lesion in about 15% of cases2. Compared with non-bifurcated lesions, the outcome of percutaneous coronary intervention on bifurcation lesions with bare-metal stents (BMS) is hampered by increased rates of procedural complications and long-term major adverse cardiac events (MACE)3. In comparison to BMS, data on the use of drug-eluting stents (DES) in the treatment of bifurcations suggest a tendency toward lower target vessel revascularisation (TVR) rates at long-term follow-up. In recent randomised studies with DES, the restenosis rate still remains less favourable than for non-bifurcated lesions, varying between 7 and 28%4-7. When restenosis occurs, it is typically found in the side branch ostium8, which might result from incomplete coverage of the ostium or suboptimal expansion at the ostium9. This may be due in part to the inability with currently available stents and techniques to provide complete stent coverage to the side branch ostium.
The Tryton™ Side-Branch Stent (Tryton Medical, Inc., Newton, MA, USA) is a balloon expandable cobalt chromium bare-metal stent, designed specifically to scaffold the ostium of the side branch. In particular, the proximal portion of the stent consists of three fronds that allow facile wiring of the main vessel. After deploying the stent in the side branch, any stent can be placed in the main branch to complete the bifurcating architecture.
The objective of the Tryton I, first-in-man (FIM) study was to test the safety and feasibility of the Tryton™ Side-Branch stent when used in conjunction with standard drug eluting stents to treat de novo bifurcation lesions.
Methods
Study design and endpoints
This first-in-man study is a prospective, multicentre, single-arm study designed to test the safety of the Tryton™ stent when used in conjunction with standard drug eluting stents to treat de novo coronary bifurcation lesions10. The study enrolled 30 patients at three participating sites. The local ethics committee for each study site approved the protocol. All patients gave written informed consent before the procedure. Patients were eligible if they had stable or unstable angina or silent myocardial ischaemia with angiography demonstrating a de novo coronary bifurcation lesion involving a main vessel with a reference vessel diameter of 2.5-5.0 mm and a side branch reference vessel diameter of 2.25-2.75 mm by visual estimate. Patients with left main lesions or bifurcation lesions previously treated with stents were excluded. Administration of aspirin and clopidogrel was mandatory up to six months.
The primary study endpoint was procedural success defined as angiographic success without in-hospital major adverse cardiac events defined as a composite endpoint of cardiac death, myocardial infarction, coronary artery bypass graft surgery or target lesion revascularisation (TLR). Clinical follow-up was performed at 30 days and at six months. Repeat angiography was planned at six months.
Study device and procedure
The Tryton™ Side-Branch Stent is a slotted tube, balloon expandable cobalt chromium BMS with three zones: a distal side-branch zone, a central transition zone and a proximal main vessel zone (Figure 1).
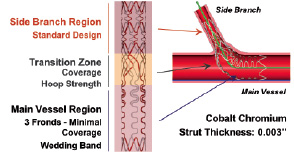
Figure 1. Schematic representing the TrytonTM Side-Branch Stent showing three zones: distal (side-branch) zone, central (transition) zone and proximal (main vessel) zone.
The distal side-branch zone has the design characteristics of a standard slotted tube workhorse stent. The central transition zone is composed of three panels, each of which can be deformed in an independent fashion. This design provides complete stent coverage of the ostium and can accommodate a complete spectrum of patho-anatomy of commonly encountered bifurcating coronary vessels. The proximal main vessel zone is composed of three fronds that are linked distally to the transition zone panels and terminate proximally in a circumferential band. The detail of the stents was described elsewhere11. The Tryton™ Side-Branch Stent used in this study (18 mm long, strut thickness of 0.003 inches) was mounted on either a straight balloon (uniform diameter of 2.5 mm) or a stepped balloon (proximal diameter of 3.5 mm, distal diameter 2.5 mm). The Tryton™ stent delivery balloons have a total of four markers: standard proximal and distal markers and two additional markers 4 mm apart delineating the transition zone.
The procedure was performed via a 6 Fr guiding catheter. Both the main vessel and side branch were wired and the lesion predilated according to the operator’s discretion. The Tryton™ stent was then positioned in the side branch with the central transition zone markers straddling the side branch origin (Figure 2).
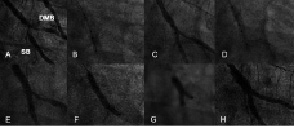
Figure 2. This is an example of a case treated with the Tryton stent. A pre-procedural cine-angiogram demonstrated severe stenosis both in the distal main branch and the side branch (A). The Tryton stent was positioned in the side branch with the central transition zone markers straddling the side branch origin (B, C) and implanted (D). Figure E is the angiogram after implantation of the Tryton stent. Following the stenting in the main branch (F), kissing balloon was performed (G). Final angiogram was shown in figure H. SB: side branch; DMB: distal main branch
After deployment of the Tryton™, the stent delivery balloon was retrieved, after which the side branch guidewire was guided through the fronds and into the distal main vessel. A standard main vessel DES was then positioned with the proximal portion placed inside the proximal region of the Tryton™ and the distal portion of the main vessel stent passing through the fronds into the distal main vessel. Selection of DES used in the main branch was left to the discretion of the investigator but limited to Taxus™ (paclitaxel-eluting stent, Boston Scientific, Billerica, MA, USA), Cypher® select (sirolimus-eluting stent, Cordis corporation, Miami Lakes, FL, USA), or Xience™ V (everolimus-eluting stent, Abbott Vascular, Santa Clara, CA, USA). After deployment of main branch DES, the side branch was re-crossed to allow simultaneous kissing balloon inflation to be performed.
Quantitative coronary angiography analysis
Quantitative coronary analysis (QCA) was analysed in an independent core lab (Cardialysis. B.V., The Netherlands). The QCA software used in this study (CAAS 5.4, Maastricht, PIE Medical software, The Netherlands) has been described separately12. In short, the analyst indicates a start point in the proximal main branch (PMB) and two endpoints in the distal main branch (DMB) and side branch (SB). Using a minimal cost algorithm, the path line between start and endpoint is extrapolated automatically (Figure 3).
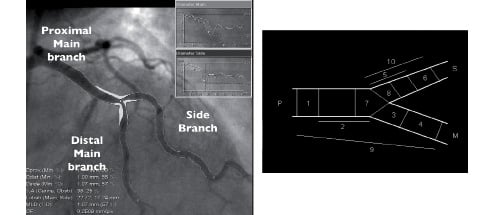
Figure 3. QCA analysis for bifurcations using CAAS 5.4. Angiographic parameters including reference diameter, minimal lumen diameter, % diameter stenosis are given in the PMB, DMB, SB(A). The 10-segment model (B) is applied for stented bifurcations. Each segment indicates the following. 1: proximal peristent (5 mm), 2: proximal main stent, 3; distal main stent, 4: distal main persistent (5mm), 5: Side branch stent, 6; distal side persistent, 7: polygon of confluence, 8: Ostium of side branch (5 mm), 9: Proximal main stent + proximal persistent (5 mm), 10: Side branch stent + distal side persistent (5 mm).
The analyst must first identify the stent boundaries so that the program can automatically divide the bifurcation lesion into a 10 segment model. For each segment angiographic parameters can be independently determined and reported. Quantitative parameters were obtained at baseline, post procedure, and at six months follow-up and reported respectively in each branch (PMB, DMB and SB). After stenting, the PMB region included the stented segment as well as 5 mm proximal to stent (equivalent to segments 1 and 2 in the 10 segment model), while DMB and SB include the stented segment and the segment 5 mm distal to stent. (equivalent to segments 3 and 4 [DMB], 5 and 6 [SB]).
Definitions
Angiographic success was defined as < 30% residual stenosis and TIMI 3 flow in both main and side branch post-procedure. Myocardial infarction was defined as either the advent of new abnormal Q-waves in accordance with the Minnesota Code for pathologic Q-Waves, or if the creatinine kinase (CK) was twice the upper normal limit with a rise in the level of the MB isoenzyme of CK (CK-MB). TLR was defined as any repeat treatment of a lesion located within the index coronary segment. TVR was defined as any revascularisation of any segment of the index coronary artery. Angiographic restenosis was defined as greater than 50% diameter stenosis on QCA within the stented segment including the stent and the 5 mm proximal and distal segments on follow-up angiography.
Statistical analysis
Continuous variables were presented as mean±standard deviation and categorical variables were presented as counts and percentages. Paired comparisons between post procedure and follow-up were done by a Wilcoxon signed rank test.
Results
A total of 30 patients were recruited into the study. Baseline clinical characteristics are shown in Table 1. The average age of the patients was 67.5 years, while 20% were diabetic and 33.3% were current smokers. Ninety-two percent of patients had angina; 56% stable and 37% with unstable presentations. Baseline lesion characteristics are presented in Table 2. The index lesion in the majority of the patients was located within the left anterior descending (LAD) artery involving a major diagonal branch. In all but one case, the procedure was performed via a 6 Fr guide catheter. Simultaneous kissing balloon inflations were performed in all cases in which the Tryton™ Side-Branch stent was deployed. A total of 39 drug-eluting stents (30 Cypher™ and 9 Taxus™) were used in the 29 lesions receiving the Tryton™ Side-Branch stent. Clinical outcomes are summarised in Table 3. Angiographic success was achieved in 96.7% and procedure success 93.3% of the cases. Two patients experienced MACE events during the index hospitalisation. The first patient had a calcified lesion involving both the LAD and a highly angulated large diagonal branch. The initial attempt to track the Tryton™ stent to the lesion site without pre-dilatation was unsuccessful. The Tryton™ stent was then removed without difficulty after which simultaneous kissing balloon pre-dilatation was performed, resulting in a coronary perforation requiring a pericardiocentesis and the placement of a covered stent that jailed the large diagonal branch. The patient was stabilised and transferred to the cardiac care unit. The patient subsequently decompensated and was brought back to the cardiac catheterisation laboratory where efforts to resuscitate were unsuccessful. The other patient having an in-hospital event had a bifurcation lesion involving the LAD-diagonal. The lesion was successfully treated with a Tryton™ stent and angiography demonstrated TIMI III flow in the index vessel. During the procedure the patient developed angina with ECG changes. Angiography demonstrated occlusion of a moderate size ramus intermedus, which was subsequently treated with stenting. Activated clotting time (ACT) obtained at that time was within normal limits. The remainder of the patients’ hospital course was unremarkable, although the CK elevation (>2 times as baseline) met criteria for myocardial infarction.
Six months clinical follow-up was obtained in all patients. The overall MACE rate was 9.9%. One patient had restenosis in the main branch proximal to the stent, which might have been caused by geographic miss in the index procedure and led to a TLR. The other patient showed progression of a residual stenosis in an untreated lesion > 10 mm distal to previously placed main vessel stent and resulted in a TVR.
QCA results
Angiographic follow-up was achieved in 23 (78%) patients. QCA results are given in Table 4. At six months, the cumulative frequency distribution curves of minimal lumen diameter (MLD) and late luminal loss (LLL) in each branch are presented in Figure 4 and 5.
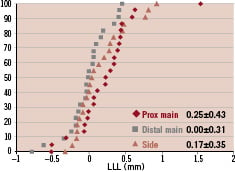
Figure 4. Cumulative curve of late lumen loss of the three divisions
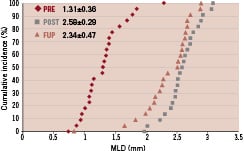
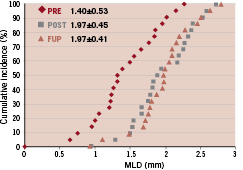
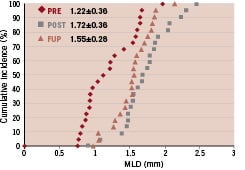
Figure 5. Cumulative MLD curve of the proximal main branch (A), the distal main branch (B) and side branch (C).
LLL in PMB, DMB, SB was 0.25±0.43, 0.00±0.31, 0.17±0.35 mm, while %DS at follow-up was 20.8±21.0. 20.5±10.2 and 18.4±9.8%, respectively. The bifurcation angles between the DMB and SB at pre, post and follow-up were 60.7°±19.1, 45.8°±14.2 and 44.7°±17.4, respectively.
Discussion
The Tryton I FIM trial assessed the safety and performance of the Tryton™ Side-Branch stent when used with a standard drug eluting stent to treat bifurcation lesions within the coronary circulation. In this trial, the Tryton™ stent has demonstrated acceptable procedural success (93.3%) and clinical safety up to 6 months after the index procedure with a MACE rate of 9.9%. Angiographic follow-up was performed in 77% of patients and demonstrated low LLL in both the main vessel and side branch (0.17 mm). A single case of angiographic restenosis was noted due to a lesion proximal to the main vessel stent (TLR 3.3%). There was no restenosis in side branch observed.
Bifurcation lesions are subject to higher rates of restenosis and more need for TLR compared with non-bifurcated lesions. Previous data from the BMS era has demonstrated TLR rates ranging from 16% to 39%13-17. The data on the use of drug-eluting stents suggests a tendency toward less restenosis with a TLR rate ranging from 4 to 31.1%4-6,8,18-20. Early data on use of sirolimus-eluting stent on bifurcation lesions suggested a higher restenosis rate after T-stenting possibly related to incomplete coverage of the side branch ostium4,21. The most effective stenting strategy for stenting bifurcation lesion remains unclear, however, as the clinical outcome of recent randomised trials using conventional stents demonstrated no clear benefit after stenting of both the main vessel and side branch (elective two stent strategy) compared with single vessel stenting (1 stent strategy)4,6,7. The Nordic Study compared a provisional 1-stent to an elective 2-stent strategy in a large (n=432) multicentre open-label randomised trial. Both groups were observed to have low TLR rates (1-stent provisional 2.9% and 2-stent dedicated 3.4%), although an increased rate of procedure-related elevation in biomarkers as well as increased procedure time was noted in the 2-stent dedicated group. Both groups had a high rate of angiographic binary restenosis (provisional 1-stent: 22.5% and dedicated 2-stent: 11.5%), which did not reach statistical significance (p=0.06)6. It is of note that these data were obtained with stents of standard design. It is unproven if drug-eluting stents which have been designed specifically for bifurcation lesions, e.g., Tryton™ Side-Branch stent, Cappella Side-Guard, Boston Scientific Petal, Abbott Vascular Frontier, will outperform standard stents22-24. In this study, the first clinical evaluation of a side branch specific stent, the 0% binary restenosis rate observed suggests that this approach is superior to both strategies tested in the Nordic Trial. This observation will need to be further validated with appropriately powered randomised controlled studies.
The SB late loss of 0.17 mm observed in the trial is less than 0.34-0.53 mm reported in the previous reports using DES4,18-20,25. The low late loss might inspire the following speculation. Firstly, drug eluted from the stent in the main branch could favourably affect the reduction of neointimal hyperplasia. Secondly, complete coverage of the side branch might reduce restenosis by preventing late recoil rather than neointimal hyperplasia, which might be further clarified by the IVUS substudy. The % DS in the SB is also low at 19%, which is comparable with other studies in bifurcations with DES (24-31% in the Nordic study). This result could be explained by a significant reduction in reference diameter at follow-up QCA, which could be related to a new method of computing reference diameter. The new bifurcation QCA software calculates the reference diameter from the healthy segment in distal side branch, so that the reference might be smaller than the one seen with conventional QCA, in which reference diameter is obtained from extrapolation of vessel size measured both in the proximal main and the distal side branch.
The approach to first stent the side branch could be controversial because it is different from the conventional approach to firstly stent the main vessel. One concern in this “SB first” approach is that the main vessel flow can be compromise by carina shift after predilatation or stenting to the SB, although this approach matches the interventional work-flow to treat the most distal disease first, avoiding the friction of the device passing through the proximal stent. Despite this concern, the FIM study demonstrated that stenting the side-branch first yielded a high angiographic success rate in 96.6% and that all workhorse stents were properly placed in the main branch by passing through the Tryton™ stent. Any difficulty in recrossing toward the distal main vessel may be related to under deployment of the frond zone which can be prevented by a proximal optimisation technique using a short oversize balloon ideally on the SB wire before removal26. Even in case the operator would experience difficulty in recrossing the distal main branch after side-branch stenting with the Tryton™ stent, the crush technique could be applied by utilising the wire in the main branch passing outside of the Tryton™ stent (Figure 6).
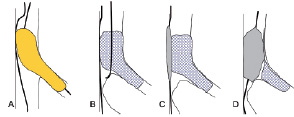
Figure 6 depicts how to retrieve the main branch after side branch stenting with Tryton stent (A) in case the operator experiences difficulty in recrossing in the distal main branch (B). The crush technique can be applied by utilising the wire in main branch passing outside of the Tryton stent (C, D).
The bifurcation angle not only affects the difficulty of the procedure but also long-term outcome. Dzavik et al reported that a bifurcation angle over 50° was an independent predictor of MACE at one year after bifurcation crush stenting in 133 patients27. This may be related to the fact that the SB stent is no more on the wall after stenting the main vessel. In the present study, 14 out of 30 lesions had a bifurcation angle over 50°, based on QCA however, the clinical outcome was acceptable. In the case of Tryton™ stent, elongation of the fronds during stenting the main vessel and kissing balloon inflation may optimise the SB stent deployment. In the study using 3D angiography by Dvir et al, they reported that the bifurcation angle is diminished after stenting (pre 71±17°vs. post 58±18°, p<0.001)28. In the present study using 2D angiography, comparison of the pre- and post- stenting angulation yielded 60.7°±19.1 and 45.8°±14.2.
A limitation of this study is that the enrolment was limited to only 30 patients. Further investigation is necessary to establish the benefit of the Tryton™ stent.
In conclusion, when used in conjunction with a workhorse DES to treat bifurcation lesions, the Tryton™ Side-Branch stent demonstrated acceptable clinical outcomes and low SB late loss with a low TLR rate at six months.

