Abstract
Drug eluting stents (DES) have attracted considerable attention due to concerns of late stent thrombosis (LST) thought to be related to delayed endothelialisation. This hypothesis is based on clinical autopsy studies indicating an association between lack of endothelium and LST. However, meta-analysis of clinical trials does not support the notion that all DES induce more late stent thrombosis than BMS. In addition, preclinical data using animal models also do not necessarily support this hypothesis. Most animal models using single non-overlapping stents show no signs of delayed endothelialisation at all. Experiments with several DES in our laboratory using the porcine coronary artery model also suggest that DES show no differences in re-endothelialisation. They can however induce (late) differences in endothelial function, depending on the DES of choice. These phenomena are also described clinically in coronary segments distal from the DES. We hypothesise that DES do not necessarily delay endothelialisation but more likely induce late endothelial dysfunction that varies between DES.
Introduction
Sirolimus (SES) and paclitaxel eluting stents (PES) have markedly reduced the rate of in-stent restenosis and late lumen loss compared to bare-metal stents (BMS)1-3, resulting in a significant reduction in the need for repeat revascularisation. However, enthusiasm for this technology has been dampened by concerns about late thrombosis (LST), an event often with serious consequences4. Delayed re-endothelialisation and endothelial dysfunction after drug-eluting stent (DES) have been suggested as the cause of LST and this has attracted considerable attention recently5. The current hypothesis is derived mainly from autopsy studies and clinical studies using intracoronary angioscopy that support delayed re-endothelialisation. However, this is still controversial, and furthermore, not all DES may induce LST (Figure 1)6.
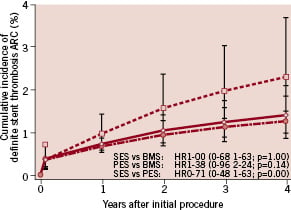
Figure 1. When plotting the incidence of definite stent thrombosis, the incidence of B6.
Our understanding of the pathophysiology of late DES stent thrombosis stems from both clinical, experimental and pathological studies. This review discusses these studies, focusing on re-endothelialisation and endothelial dysfunction. In addition, we present our perspective derived from experience with stented porcine coronary arteries.
Animal studies
In porcine coronary arteries early re-endothelialisation is usually studied at five days after stenting. A time point early enough to assess differences in healing before endothelialisation is complete, mostly using scanning EM and computerised planimetric analysis7. To understand the role of the endothelium we restudied tissues from the first generation BMS, the Palmaz-Schatz stent in comparison to the newer designs8. We found significant differences in the percentage endothelialisation of the stent struts at five days in the bare Palmaz-Schatz stent (60±27%; n=10) and in the bare divYsio stent (91±12%; n=10, p<0.05, T-test). While this difference is quite large, it had no effect on the amount of intimal thickening at 90 days, being 0.2±0.05 mm and 0.2±0.1 mm respectively7,8. It is not surprising that early presence of endothelium does not affect healing at 90 days as BMS have been known to interfere with endothelial function and induce chronic injury9,10. It raises the question whether this is different in DES. Indeed, current DES yield similar endothelialisation rates to BMS (Figure 2; 82±1, 80±22 and 83±25 in tacrolimus, sirolimus and paclitaxel eluting stents, versus 93±8% in BMS, ANOVA, p=0.6).
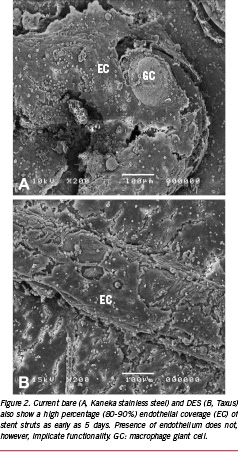
While this contradicts the current hypothesis of delayed endothelialisation, our data are in accordance with other animal studies. In a study by Klugherz11 using SES in rabbit iliac arteries, little evidence of increased inflammation and delayed endothelialisation was noted with 28-day SES as compared to polymer-coated stents or BMS. And although Suzuki12 reported higher amounts of accumulated fibrin with SES than BMS at 28-days in porcine coronary arteries, the degree of re-endothelialisation was similar among groups. Indeed, most papers describe a fairly high rate of endothelialisation in single DES and BMS that appear strikingly similar (80-90%). Only in segments of overlapping DES with presumably high local drug levels, can an effect be seen (60-70%).13 Overlap stenting is however rare in daily practice (0.9%) and although in both BMS and DES this is associated with a high rate of revascularisation14, overlap stenting has not been identified as a predictor of LST15.
Absence of neointima cannot be ascertained by angioscopy
It should be noted that in clinical studies it is especially difficult to determine the difference between lack of endothelialisation, endothelial contraction and endothelial dysfunction. Several clinical angioscopic studies have described delayed endothelialisation after implantation of DES with SES showing incomplete neointimal coverage (NI) three to six months after implantation. This was associated with presence of thrombi and yellow plaques even as much as two years after implantation16-18.
We doubt whether angioscopy can really distinguish complete from incomplete endothelial and thin NI-coverage. In swine coronary arteries, we have often observed stents that look like non-covered stents as seen by macroscopy (Figure 3). Light and scanning electron microscopy however show that these stents are not at all naked, but completely covered by endothelium as well as neointimal tissue. This tissue is however completely translucent to the extent that red thrombus can be easily observed surrounding stent struts10.
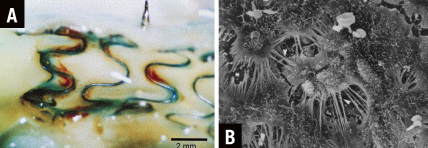
Figure 3. Although stent struts have the macroscopic appearance of a completely non-covered Medtronic Wiktor stent at 14 days after stent implantation (angioscopic grade 0, stent struts fully visible16 [A]), scanning electron microscopy shows that the struts are actually completely covered by endothelium (right, modified from van Beusekom et al10 [B]).
Autopsy studies
Autopsy studies in humans have advocated the link between delayed endothelialisation (delayed healing) and LST, showing an especially strong association between lack of neointimal strut coverage in DES and LST15,19,20. Markers of delayed healing have also been observed in atherectomy specimen in a study that specifically excluded stent thrombosis21 and seems unrelated to LST. Of course both approaches have clear drawbacks. Atherectomy specimen represent cases of restenosis and pertain to small pieces of tissue, while autopsy cases only cover a discrete subpopulation of DES implantation and complete clinical data are not always possible to obtain. With autopsy, there is also an inevitable delay between the time of LST and tissue retrieval at autopsy. Whether the endothelium underneath a thrombus, often rich in neutrophils, can survive this delay and remain present for assessment is unclear22. This cautions us to apply this data to all “real-world” cases of stent thrombosis.
Endothelial dysfunction: abnormal response to vasodilators or exercise
If mere presence of endothelium does not affect healing, assessment of endothelial function seems more appropriate and can be studied both in vivo and in vitro. Indeed, recent clinical reports suggest that DES may impair endothelial responses to acetylcholine (Ach) or exercise-mediated vasodilation in humans23-26.
Hofma and Shin both showed that coronary segments adjacent and distal to DES showed vasoconstriction in response to Ach in SES but not BMS (Figure 4), both without impaired endothelial independent vasodilator responses to nitroglycerin. Endothelial dysfunction was corroborated by experimental studies in swine showing impaired dilation in response to Bradykinin which was more evident in PES than SES27. Togni evaluated coronary vasomotion with quantitative coronary angiography at rest and during exercise in patients receiving SES, PES and BMS at six 2-12 (two to twelve) months after implantation. Both SES and PES showed exercise-induced vasoconstriction of the proximal and distal vessel segments adjacent to DES, while in BMS, the adjacent segments proximal and distal to the stent showed exercise-induced vasodilation. They concluded that DES are associated with exercise-induced paradoxic coronary vasoconstriction of the adjacent vessel segments, despite a maintained vasodilatory response to nitroglycerin.
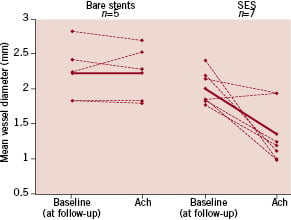
Figure 4. Sirolimus eluting stents induce chronic endothelial dysfunction in the coronary segment immediately distal from the stent as shown by a vasoconstrictive response to an acetylcholine (10–6M) challenge at follow-up (P=0.03). From Hofma et al23.
Endothelial function within stents themselves is most easily studied in animal models using immunohistochemistry. Our data from the animal lab also indicate that DES are not so much associated with endothelial absence but more with a more pronounced endothelial dysfunction as shown by a decrease in eNOS expression in Paclitaxel but not Sirolimus eluting stents as compared to BMS28.
Endothelial dysfunction or endothelial absence
Acetylcholine acts as a vasodilator by stimulating the release of NO by the endothelium. An abnormal Ach test is usually interpreted as a form of endothelial dysfunction. However, endothelium that has a normal NO release but is contracted and thus leaky to many substances (Figure 3B)10,29, can probably also cause vasoconstriction via a direct exposure of smooth muscle cells to Ach. In that sense, it is not easy to distinguish between diminished NO release, endothelial leakage or absence of endothelium. The absence of endothelium in vivo is nearly impossible to assess in clinical research.
Conclusion
Although current hypotheses propose delayed endothelialisation after DES implantation, evidence is still controversial. Both clinical studies as well as our own data from in vivo and in vitro porcine coronary studies27, suggest that DES do not necessarily show differences in re-endothelialisation. They do underscore that there are late effects of stents, DES in particular, on endothelial function. These effects could well be responsible for a pro-thrombogenic nature in some but certainly not all DES or BMS.

