Abstract
Aims: To study how the balance between tacrolimus elution and polymer degradation from drug-eluting stents (DES) affects neointimal thickening (NIT) in swine coronary arteries.
Methods and results: We assessed a fast-degrading high dose (2 µg/mm2), a slow degrading low dose (1 µg/mm2) or polymer-only coated DES (Pol) versus bare metal stent (BMS). Coronary segments were pre-injured with a balloon/artery ratio of 1.1 to 1.3. Then stents were implanted at that site with a stent/artery ratio of 1.1, with a follow-up period of 5 to 180 days. Histology showed a well endothelialised neointima (82±1% in high dose DES vs. 93±8% in BMS) already at five days, without differences in eNOS expression. Morphometry indicated that neointimal thickness in DES was significantly reduced as compared to BMS and Pol at 28 and 90 days. Polymer degradation products induced a distinct inflammatory response which was effectively suppressed in DES. Between 90 and 180 days, however, the slow degrading low-dose stent showed catch-up of NIT.
Conclusions: Tacrolimus eluted from a biodegradable stent coating can suppress the inflammatory effect of the coating degradation products if the balance between the drug levels and the degradation products is favorable.
Introduction
The introduction of drug eluting stents (DES) has markedly reduced the need for repeat revascularisation compared to BMS (bare metal stents) in patients with coronary artery disease.1,2 The efficacy of DES in reducing NIT, however, varies considerably. Angiographic late loss in clinical trials with DES differs considerably with values varying between 0 and 1.40 mm and likely due to a different balance between pro- and anti-proliferative stimuli. This can be caused by the drugs, the elution rate of the drugs as well as by polymer coatings or, in case of degradable coatings, by their degradation products. For the latter, the balance between drug release and the irritant effects of the polymer degradation needs to be carefully tailored.
In the present study we tested the hypothesis that the efficacy of tacrolimus eluting from a completely biodegradable PLGA (polylactide-polyglycolide polymer) coated stent to reduce neointimal thickening (NIT), depends on the balance between the anti-proliferative and anti-inflammatory effects of tacrolimus and the pro-inflammatory effects of the polymer degradation products. We tested this hypothesis in a swine model of vascular injury and stent implantation, with a follow-up period long enough to allow complete coating degradation of drug-loaded and non-loaded polymer coated stents.
Materials and methods
Animals
Experiments were performed in farm-bred swine (Yorkshire-Landrace, 30-35 kg) and Yucatan miniswine (45-55 kg). The study was approved by the animal ethics committee of the Erasmus MC and complied with the regulations of the “Guide for the care and use of laboratory animals” (NIH publication 85-23).
Injury model
We used oversized balloon angioplasty prior to stenting to induce vascular injury. By damaging the artery with a balloon to artery ratio of 1.1 to 1.3, a range in injuries varying from no apparent injury to rupture of the internal elastic lamina or even medial rupture is induced. This will result in a vascular healing and inflammatory response that can unmask biocompatibility problems of the polymer carrier that remain invisible when subjected to a vascular healing environment of minimal injury. By refraining from stent induced oversizing, shear stress changes are not introduced as a covariant influencing NIT4.
Polymer
The coating consists of polylactide-polyglycolide (PLGA) polymer. The degradation profile of the polymer coating with incorporated drug was measured by Kaneka Corp. to be complete at 180 days.
Tacrolimus
This macrolide produced by Streptomyces tsukubaeniss, has strong immunosuppressive actions5. It is highly lipophilic and traverses the cell membrane without dependence on cell-surface receptors. The intracellular receptors or immunophilins are the FK binding proteins (FKBP, including FKBP12). The T-immunophilin complex binds with high affinity to inhibit the calcineurin-calmodulin complex6. It inhibits platelet and neutrophil recruitment, expression of endothelial adhesion molecules, free radical- and inflammatory cytokine release7.
Stents
BMS, Pol and two DES: low dose (TL, 1 µg/mm2, degraded in 110 days) and high dose (TH, 2 µg/mm2, degraded in 90 days) are based on a 316L stainless steel balloon-expandable coronary stent (length 13 mm, diameter 3.0 and 3.5 mm, Kaneka Corp., Osaka, Japan).
Animal preparation was performed as described before18. In short, animals were pretreated p.o. with 300 mg acetylsalicylic acid and a loading dose of 300 mg of clopidogrel (Plavix, Sanofi Aventis, Gouda, The Netherlands) one day prior to the procedure. After induction of anaesthesia and connection to the ventilator, antibiotic prophylaxis was administered by an intramuscular injection of streptomycin, penicillin procaine (0.1 mg/kg). An introducer sheath was placed in the carotid artery for arterial access. A dose of 250 mg ASA and 10.000 i.u. heparin was given (i.a.).
Early endothelialisation
A small group of Yorkshire swine (n=3) was used to test the effect of T on early stent endothelialisation. Cobalt-chromium (CoCr) T-eluting stents (75 µm strut thickness, 2 µg/mm2 T, CoCr TH) and CoCr BMS stents were implanted as described above (stent/artery ratio 1.1). Five days later animals were sacrificed, and SEM was performed on the stented segments18.
The tissue response to BMS, Pol, TL and TH stents was studied at three time points (Table 1): 28 days (maximal NIT), 90 days (regression of NIT, near complete polymer degradation in TH) and 180 days (complete polymer degradation in TH, TL and Pol).
The 28 day (n=12) and 90 day follow-up (n=12) was performed in Yorkshire swine. Fast-growing farm bred swine are less suited for long follow-up studies, and therefore the 180 day study was performed in Yucatan miniswine (n=16). For interspecies comparison of tissue responses to stenting, five additional Yucatan miniswine were studied at 90 days.
Balloon injury, stent implantation and angiography
Balloon injury and stent implantation were performed as described before8,9. Under guidance of quantitative angiography (CAAS II, PIE Medical, Maastricht, The Netherlands), balloon injury was inflicted with an angioplasty balloon inflated to achieve a balloon-artery ratio of 1.1, 1.2 or 1.3:1. Thereafter, the stent was introduced at the same site but with a balloon-artery ratio of 1.1:1. All animals received three different stents each, one per coronary artery, according to a predetermined randomisation scheme for balloon injury and stent type. After the final angiogram, the animal was allowed to recover from anaesthesia and returned to the animal care facilities for the post operative recovery. During the follow-up period, 300 mg ASA and 75 mg clopidogrel was administered daily p.o.
Follow-up study
At follow-up animals were anaesthetised, and quantitative angiography was performed followed by euthanasia through an overdose of pentobarbital. Hearts were pressure fixed in situ using 500 ml 4% buffered formaldehyde in preparation for histology and immunocytochemistry8.
SEM
After fixation with buffered 4% paraformaldehyde and 2,5% glutaraldehyde, the arteries were processed as previously described10. Endothelial regrowth was studied along the length of the stented segments on the stent struts. The endothelium was marked and the percentage endothelial coverage of the stent struts quantified using computer assisted planimetry.
Histology
The arteries, including 1 to 2 cm both proximally and distally adjacent to the stent, were processed for plastic embedding9,11. Sections were collected of the non-stented adjacent artery, and at three levels (the proximal, middle and distal part) in the stented segments and stained with Hematoxylin-Eosin and Resorcin Fuchsin (elastin stain) for quantitative and qualitative analysis.
Immunocytochemistry was performed in the mid-stent region using the lectin BSIb4 (Sigma-Aldrich, Zwijndrecht, The Netherlands) as an endothelial marker and antibodies against smooth muscle α-actin (clone 1A4, M0851, Dako, The Netherlands), leucocytes (MCA 1447, Serotec, Kidlington, UK), macrophages (MCA874, Serotec, Kidlington, UK), eNOS (sc-654, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and vWF (A0082, Dako, The Netherlands). A semi-quantitative analysis of the eNOS, vWF and lectin expression was performed: positive endothelium was presented as % of total covered lumen circumference, receiving scores between 0 and 5: 0=0 positive cells, 1= 0-10%, 2=10-40%, 3=40-70%, 4=70-90%, 5=90-100%.
Morphometry, injury score and inflammatory score analysis was performed as described before10,12. Inflammation was determined by presence of inflammatory cells surrounding the stent struts, no inflammation being defined as no inflammatory cells around the struts and full inflammation meaning that all struts proximal, middle and distal were surrounded by inflammatory cells.
Statistical analysis
All data are given as mean±SD and were analysed with SPSS (SPSS Inc. version 11.5.0). Intergroup differences were initially assessed using ANOVA. Statistical significance was considered for P<0.05. In case of differences between groups, further testing was performed using linear regression analysis. For angiography the main model parameters consisted of species, follow-up time, balloon/artery ratio, stent-artery ratio and stent type. For morphometry the main model parameters were the same but also included morphologic injury score. Every regression analysis includes ANOVA to test statistical significance of the model.
Results
A total of 137 stents were successfully placed in the coronary arteries of 50 swine. All animals survived the follow-up period. At sacrifice, no macroscopic evidence of stent thrombosis or myocardial infarction was seen.
Quantitative angiography, summarised in Table 1, showed no differences in vascular diameters pre- or post- implantation between groups (p=0.4 and 0.9 resp.), nor differences in balloon injury and stent-artery ratio between the groups (p=0.5 and 0.2 resp.). There was a significant difference in overall late lumen loss (using all time points) between Pol and TL which was independent of balloon injury (regression analysis, 0.16±0.3 vs. 0.04±0.3 mm, p=0.03) and a trend towards a difference between Pol and TH (0.16±0.3 vs. 0.08±0.3 mm, p=0.099).
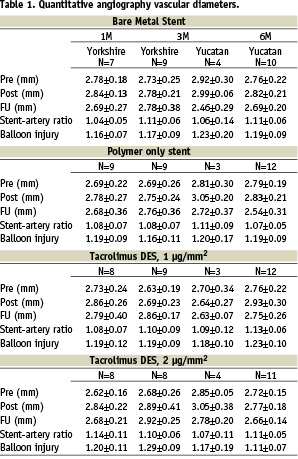
SEM indicated that as early as five days, in both TH and BMS there was almost complete re-endothelialisation (82±1.4% vs. 93±8%, p=0.09) which was confirmed by immunocytochemistry, indicating that the highest T concentration was not toxic for the endothelium (Figure 1).

Figure 1. Overview and details of SEM of TH (A, C) and BMS (B, D) showing stent struts covered with endothelium at five days following implantation. All stent struts are only partly covered by endothelium, the areas not covered by endothelium often reveal macrophage giant cells or protein-covered stent struts.
Qualitative histology showed complete endothelialisation in all groups at 28 days. At this time, the NIT consisted mainly of smooth muscle cells within a collagenous matrix, containing scarce inflammatory cells. This was similar at 90 and 180 days (Figure 2).

Figure 2. Histology of BMS (A, B), Polymer (C,D), TL (E,F) and TH (G,H), at 90 days (left column) and 180 days (right column), showing the similarity in appearance in all groups. H&E stain, Bar=1000 µm.
In Pol however, we observed more inflammatory cells near the stent struts at 28 days, and an intense inflammatory response towards the polymer at 90 and 180 days (Figure 3A, B, full inflammation in 50% of vessels).
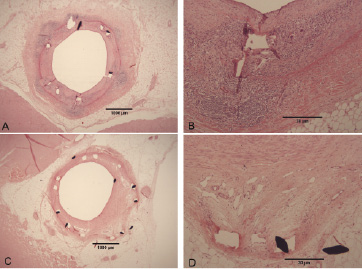
Figure 3. Active (A,B) and resolved (B,C) inflammation in a polymer coated stent, showing a granulomatous reaction (A,B) with macrophage giant cells, even in the adventitia (arrow), and extensive neovascularisation in a case of resolved inflammation (C,D, arrows).
In some Pol stents, the presence of extensive neovascularisation without inflammation indicated that an inflammatory response had died down completely at 180 days (Figure 3C, D). This indicated that inflammation was a late occurring phenomenon, starting between 28 and 90 days, which was corroborated by the presence of a well organised NIT covered by endothelial-like cells, while inflammation was only seen deep in the NIT.
At all time points we observed a limited amount of proteinaceous material (fibrinoid) surrounding stent struts. This involved 10-30% of struts at 28 days in all groups, including BMS (Figure 4).
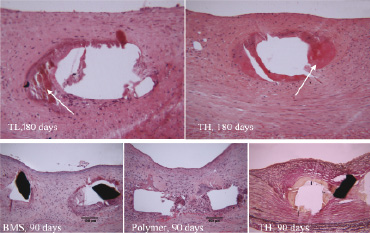
Figure 4. Proteinaceous deposits (fibrinoid) and small areas of calcification surrounding the stent struts were observed in all groups, even in bare.
This prevalence increased to 20-50% at 90 days and to 30-65% at 180 days, again being similar in all groups.
Endothelial immunocytochemistry
Qualitative assessment at five days showed endothelium positive for eNOS, while vWF activity was mainly restricted to the intima. Difficulty in procuring sections with complete intact intima and endothelium did not allow further quantification. At 28 and 90 days, morphologic appearance and a high level of lectin expression, similar for BMS, Pol, TL and TH (score above 4 in all groups), indicated completeness of the endothelial layer. A trend towards an increased expression of eNOS (Figure 5) was found in TH (Score: 4.40±0.55) compared to Pol (3.38±0.92) at 90 days (P=0.06 by ANOVA), consistent with an improved endothelial function in TH. In all groups vWF expression was very low (scores ~ 1-1.5), both at 28 and 90 days.
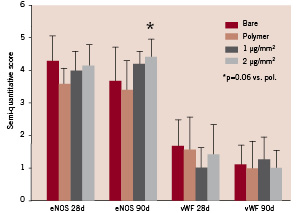
Figure 5. Semiquantitative assessment of endothelial functionality at 28 and 90 days through assessment of eNOS and vWF expression. Error bars are 1 SD.
Morphometric analysis is summarised in Table 2 and Figure 6.
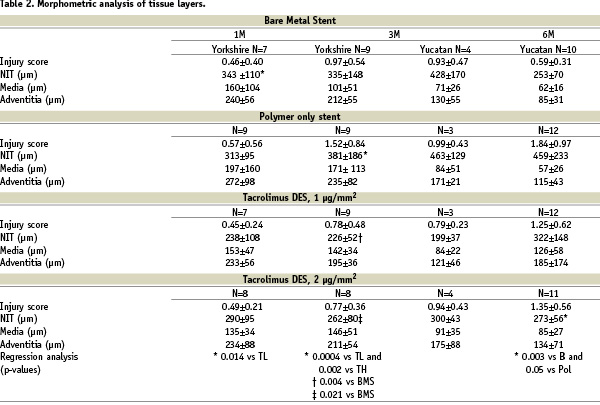
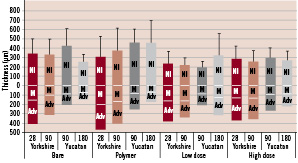
Figure 6. Morphometric analysis of the total wall thickness (i.e. NIT+ media (M) + adventitia (Adv)) measured on elastin-stained sections taken from the proximal, medial and distal region of the stent.
Data show that all stent groups show a regression in total wall thickness, (NI+M+Adv), over time except for the TL. When corrected for injury, both drug eluting stents showed an overall highly significant decrease in NIT as compared to BMS (p=0.003). Subgroup analysis per time point showed that at 28 days there was no difference in NIT between stents. At 90 days there was a significant decrease in NIT in the DES compared to the BMS and Pol (Table 2). At 180 days only TH was significantly lower as compared to BMS and Pol stent while no statistically significant difference was seen between the TL, BMS and Pol stents.
Injury scores are summarised in Table 2. At 28 days, the injury scores are very similar in all groups. There is however a clear increase in injury scores over time in all groups containing polymer, with or without drug, but highest in Pol (p=0.00004, 0.002 and 0.006 vs. B, TL and TH). At 180 days, the slope between injury and NIT in TH was significantly blunted (Figure 7).
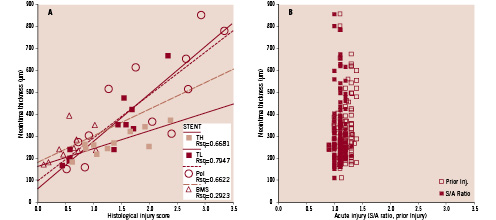
Figure 7. A. The neointimal response to histological injury at 180 days shows that TH blunts the neointima thickening while TL and Pol show a similar response. B. Acute injury (S/A ratio, Prior injury) inflicted to the vessels at implantation does not correlate to neointima thickness (R2=0.0145 NI vs Prior injury; R2=0.0002 NI vs S/A ratio). Since there was no difference between the different stents, data from all stents at all follow-up time points were pooled.
Inflammation at 28 days showed that full inflammation was observed in one Pol stent only. At 90 days however, full inflammation was now observed in five out of nine polymer stents but only one out of nine TL stents. At 180 days the inflammatory response to Pol remained constant (three of six vessels), while BMS, TL and TH group showed minimal inflammation.
Discussion
Angiographic late loss in clinical trials with DES differs considerably3 with values varying between 0 and 1.40 mm. This can be caused by the drugs, the elution rate of the drugs, the polymer coating or, in case of degradable coatings, their degradation products. It is clear that the balance between drug release and the irritant effects of the polymers or their degradation products needs to be carefully tailored.
Polymers, as any other implant, induce the formation of a fibrous cap. In case of PLGA, slow degradation and thus longer presence have been correlated with an increased fibrous cap thickness and a prolonged inflammatory response as compared to fast degradation13. The drugs eluting from DES can attenuate the formation of this fibrous cap. Efficacy may depend on the duration and degree of drug release and polymer degradation. Obviously, the drug should not induce unwanted side effects such as interference with vascular healing and function as a present and functional endothelium is critical14-17.
Cell culture studies have indicated that drugs such as Sirolimus and paclitaxel reduce endothelial proliferation. In contrast to Sirolimus which shows a higher affinity for endothelium than smooth muscle cells, tacrolimus has less affinity for endothelium than smooth muscle cells18. This makes it a candidate for a DES to reduce intimal thickening without affecting re-endothelialisation or endothelial function.
We studied a novel Tacrolimus eluting stent based on PLGA with a variable degradation rate and drug release profile. The follow-up time extended beyond the time of complete polymer degradation in both drug and polymer control groups, to identify possible late undesirable events.
Our main findings are that Tacrolimus allows for early and functional re-endothelialisation, since as early as five days we observed almost complete coverage with functional endothelium, comparable to BMS. Secondly, this resulted in a persistent and long-term reduction in NIT as compared to BMS and Pol without delayed healing or inflammation. These results indicate that Tacrolimus can effectively reduce NIT following stenting, while effectively abolishing a response towards the polymer degradation products. In particular, late build-up between 90 and 180 days in TH was not observed, likely because the polymer coating had already completely degraded.
Polymer degradation and drug release
At several time points, up to 84 days, a total of three stents per time point were assessed with respect to degradation of the polymer coating, drug release and arterial drug uptake. A forward projection of the fitted degradation curve (Figure 8) indicated that the time required for total polymer degradation was 90 days for TH, 113 days for TL, and 131 days for Pol.
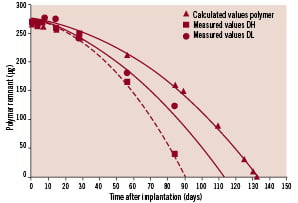
Figure 8. The measured polymer degradation rate for the DL and DH coating, and the predicted degradation rate for the pure polymer coating. Each measured point is an average of three stents in one animal. Pure polymer degradation is estimated with the assumption that the degradation rate has a linear dependency on drug concentration. The forward prediction is based on regression analysis, and shows the timepoint of completed polymer degradation.
This difference in degradation rate is caused by increased porosity of the polymer coating following drug release. Thus, up to 90 days the wound healing processes in TL, TH and Pol are determined by the combined effect of drug and polymer which are simultaneously released during degradation. After 90 days, the polymer coating of TH has completely degraded and all T released, while in TL and Pol, the degradation of the polymer is still continuing but without sufficient T to dampen the tissue response.
Neointimal build-up, a balance between drug release and polymer degradation
Our data indicates that there is a balance between the neointimal response and the patterns of drug release and polymer degradation in time. Thus, in response to Pol, the neointima thickness appears to increase in the first 90 days, possibly due to a reaction towards the polymer and the polymer degradation products, as an increased inflammatory response and a tendency towards reduced eNOS expression was also seen in this group as compared to the other stents. The low dose (1 µg/mm2) degrades slowly resulting in low levels of degradation product over 110 days. The drug released over the same time, was half of the high dose and unable to prevent a late catch-up: not enough drug for the amount of polymer degradation product irritating the vessel wall.
The high dose (2 µg/mm2) degrades fast thereby releasing relatively high levels of degradation product in a short period. The drug, released in a shorter period than the low dose, was thus more than twice the low dose and prevented the catch-up “polymer effect” as the stent is basically a bare stent at 90 days. The polymer-drug combinations in our study do not allow independent analysis of the individual components, but that does not preclude assessment of the balance between degradation rate and drug release, especially because the two designs show such a difference. The only conclusion is that the right balance between polymer degradation product and drug levels can reduce maximal intimal thickening.
Model of vascular injury
Injury scores are often used as an index of mechanical injury to be correlated to, and correct for, the intimal response. We used a model of graded vascular injury prior to stenting to ascertain both a best case and worst case scenario for the polymer coated devices. This was thought to be of importance especially as vascular injury seemed to unmask polymer incompatibility19. Our current data using S/A ratio and prior injury do not support this hypothesis anymore. Figure 7B clearly shows no relation at all between acute balloon injury, stent-artery ratio and the resultant neointima. Figure 7A however shows that the histology based injury score at follow-up, as a measure of chronic injury20, does correlate with neointimal thickness. Here the high dose Tacrolimus seems to blunt this effect. This effect may potentially be even stronger when future attempts towards the use of more biocompatible polymer formulations are successful.
The morphologic injury score in BMS was very low, much lower than historic data20. It is attractive to think that this is a result of improvements in stent design over time. The higher increase in morphologic injury in time for the coated stents as observed in our data indicates that aside from the chronic mechanical injury, there is another component involved in this increase. It is unclear whether this is purely the result of the local chemistry (i.e. polymer degradation products) or whether the surface changes (increased roughness) that go hand in hand with coating degradation are responsible. Importantly however, it implies that we should be cautious when using morphologic injury scores at later time points, especially in conjunction with polymeric coatings.
Endothelial function
The current drug eluting stent was shown not to interfere with early re-endothelialisation, showing a high percentage endothelial coverage of the stent struts at five days, similar to BMS. In addition, we showed that this endothelium was also functional in producing eNOS and more importantly, no increased expression of vWF, indicating that the endothelium was not pro-thrombogenic. A fully functional endothelium without an activated state is an important feature in any stent. This stent-drug-polymer combination seems not to interfere with this phenotype.
Earlier Tacrolimus-eluting stent studies
Some experimental stent studies, using varying coatings and T concentrations, have shown efficacy at 28 days21-23, but not all24. In contrast to the current study, they all failed however to demonstrate efficacy at 90 days, likely due to a deficient balance between drug delivery profile (fast release) and coating induced inflammation.
In conclusion, the fast-release high dose tacrolimus eluting stent (2 µg/mm2) successfully reduces in-stent neointimal thickening up to 180 days, in coronary arteries following balloon injury, without interfering with wound healing and allowing early re-endothelialisation.
Acknowledgements
The technical assistance of W. Kerver, BSc, I. Peters, BSc, S. Swager-Ten Hoor, R.N. and S. Kazim, BSc, is gratefully acknowledged.

