The commonest reason for failure of recanalisation of a chronic total occlusion (CTO) is failure to cross the lesion with a guidewire. However, over recent years, there have been huge developments in percutaneous coronary intervention technologies, most importantly with the introduction of specialised CTO guidewires. In addition, several techniques to improve recanalisation success rates have been developed, particularly in Japan, and are outlined in the article by Dr. Mitsudo. The aim of the present paper is to try to understand why we approach the percutaneous therapy of chronic total occlusion therapy the way we do.
Introduction
Chronic total occlusions (CTOs) are commonly found on diagnostic coronary angiography. In one recently published large registry of more than 6,000 patients, at least one CTO was found in 52% those with significant coronary disease (defined as a diameter stenosis of >70%).1 The major limitation of a successful outcome to the intervention of a coronary chronic total occlusion (CTO) is the inability to cross the lesion with a wire.2,3 This difficulty has a major impact on the therapeutic decision making of the cardiologist. In published studies, the presence of a CTO on coronary angiography has meant that these patients have been significantly more likely to be managed with either medical therapy or referred for coronary artery bypass graft surgery.1,4
Successful percutaneous intervention of a CTO has important implications for the patient, leading to improved anginal symptoms, improvement in left ventricular ejection fraction, and a reduced need for CABG surgery.2,5-11 Recently the improvement in left ventricular function has been shown to be predictable by pre-operative assessment of myocardial viability with MRI.12 Importantly, large registry data has shown that in addition successful CTO recanalisation confers a long-term survival advantage compared with those who have an unsuccessful attempt at CTO angioplasty.10,13 Historically, CTOs have been associated with a relatively high rate of restenosis. However, drug-eluting stents have demonstrated efficacy in these lesions with low rates of restenosis (2-11%) and re-occlusion (0-4%), further improving the long-term outcome of these patients.11,14-19
Predictors of successful CTO recanalisation
Multiple angiographic features have been suggested to be predictive of percutaneous recanalisation failure2 including a longer length of occlusion, longer duration of occlusion, presence of calcification, presence of bridging collaterals, a blunt (as opposed to tapered) stump, presence of a side branch at the site of occlusion, and vessel tortuosity. In recent years, there have been significant improvements in wire technology, with the development of several specialised wires dedicated to CTO therapy. In accordance with this, in experienced hands, CTO recanalisation success rates have also increased, and the presence of these angiographic adverse features, many of which were described more than 10 years ago, should not necessarily deter an attempt to recanalise a CTO percutaneously. A recent study evaluated the use of pre-operative non-invasive imaging with multi-slice computed tomography (MSCT) scanning, in addition to the “traditional” angiographic features, to predict angioplasty outcome.20 In this study of 49 patients, MSCT did provide additional information. By multivariate analysis, there were 3 predictors of CTO recanalisation failure: presence of a blunt stump as seen on angiography, length of occlusion >15 mm (as measured on CT scanning), and the presence of heavy calcification (as detected on CT scanning).
Lessons learnt from pathophysiology
Histological examination of CTOs has been important in our understanding of these lesions and the development of techniques to improve recanalisation rates. A relatively recent occlusion is composed of soft or lipid laden material making penetration and crossing of the occlusion easier. The older an occlusion becomes, the more organised it is, with increasing degrees of dense fibrous collagen and calcification (Figure 1).21-23
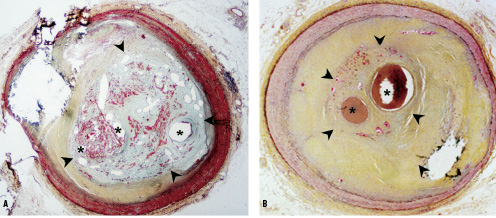
Figure 1. Human coronary arteries with healing (panel A) and healed (panel B) total occlusion. Panel A, shows a healing total occlusion represented by the inner boundaries of the arrowheads. There are multiple vascular channels (asterisks) of varying sizes embedded within a provision matrix characterized by fibrin (red) and proteoglycans (bluish-green) staining. Panel B, represents a healed total occlusion (arrowheads) with vascular channels (asterisks) surrounded by a rich collagen matrix (yellow). Both sections were stained with a modified Movat Pentachrome (20 magnification). Image courtesy of Drs Frank D. Kolodgie and Renu Virmani, Department of Cardiovascular Pathology, Armed Forces Institute of Pathology, Washington, DC, USA.
CTOs occur following rupture of an atherosclerotic plaque with bidirectional thrombus formation. This does not necessarily present acutely as a myocardial infarction, as the presence of a long-standing significant stenosis may be associated with the development of collaterals from other arteries, which supply blood to the vessel distal to the occlusion. With time, the relatively soft thrombus and lipid pool is replaced by collagen, with particularly dense fibrous tissue evident at the proximal and distal ends – the so-called “caps”. In addition, particularly after the first year, the lesion becomes increasingly calcified.
Histology has also shown evidence of neovascularisation with microvessels that cannot be seen on contrast angiography, which has a resolution of approximately 250 µm. Indeed, more than 75% of angiographically demonstrated “occlusions” are not actually completely blocked on histology. Microvessels form the vasa vasorum are seen in the adventitia and outer media, and tend to run radially. If well developed, these can be recognised as “bridging collaterals”. Neovascularisation also occurs within the atherosclerotic plaque, and within the thrombus as it becomes organised. These latter microvessels are generally 100-200µm though can be as large as 500 µm.24 They are particularly important in CTO recanalisation as they run parallel to the occlusion, and may therefore provide a path for the guidewire (an 0.014” wire is 360 µm). Strauss et al have recently reported their results of high resolution imaging of these microvessels in a rabbit femoral artery CTO model, using MRI and three-dimensional micro-CT. MRI techniques can provide a spatial resolution of down to 100-200 µm in-plane. The new technique of micro-CT is performed ex-vivo on excised vessels, using a low viscosity polymer compound (Microfil) that fills the microvessels. Micro-CT imaging provides evaluation of these microvessels to a resolution of 17 µm (Figure 2).

Figure 2. Micro-CT of a 24-week old chronic total occlusion (rabbit model) demonstrating development of extensive microvessels. Image courtesy of Dr. Bradley Strauss, St. Michael’s Hospital, Toronto, Canada.
There are therefore 4 important components of the occlusion to take into consideration:
– Dense fibrous tissue at the proximal cap
– Calcification
– Microvessels
– The distal cap
The proximal cap
Because of the dense fibrous tissue, penetration of the proximal cap can be difficult particularly if the occlusion stump is blunt. In addition, if a side branch is present at the site of occlusion, then guidewires “take the path of least resistance”, and tend to be deflected into the branch (Figure 3).
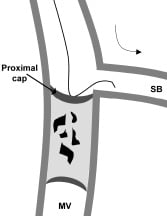
Figure 3. The dense fibrotic tissue of the proximal cap makes penetration difficult; the wire simply prolapses into the side branch. MV: main vessel, SB: side branch.
Specialised CTO guidewires have relatively stiff tips that can penetrate the cap; in addition, some also have a tapered tip. It is important to understand the stiffness of guidewire tips that are graded in terms of grams – the tip load being the weight needed to bend the tip. One example is the family of Miracle wires (Asahi Intecc, Japan), which are available in 3 g, 4.5 g, 6 g and 12 g. A standard floppy wire has a <1 g tip, an intermediate tip wire is ~3 g, anything above this is particularly stiff and such wires should be used with care. Examples of tapered tip wires are the Conquest (Confianza) wire (Asahi Intecc, Japan), which is a 0.014” wire with a tapered tip of 0.009” and a tip load of 9 g, and the Cross-it wires (Guidant Corporation) which have a tip of 0.010” and come in a range of stiffness. Further increase in penetration capability can be attained from using a supportive guide catheter, and wire support with an over-the-wire balloon or micro-catheter (Figure 4).
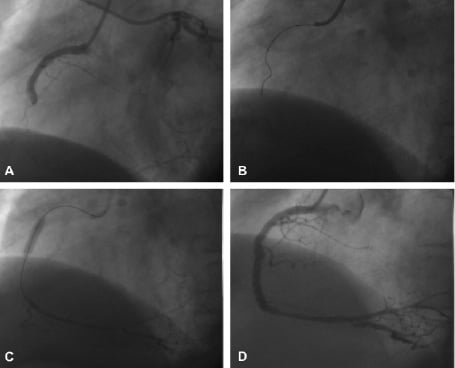
Figure 4. Case example: A 64-year old man with a 2-year history of stable angina. Coronary angiography demonstrated single vessel disease with complete occlusion of the right coronary artery (A), left ventricular function was normal. A Judkins right guide catheter was used, with the distal vessel filled from simultaneous left coronary artery injection. The proximal cap proved to be very difficult to penetrate; initial attempts using a Miracle 3 g wire (Asahi Intecc, Japan) with over-the-wire support were unsuccessful (B). Guide catheter support was inadequate, so a compliant 3.0mm balloon was inflated to nominal pressure in the proximal vessel to “fix” the catheter and increase penetrability of the wire. Success was achieved with a Miracle 4.5 g wire (C). This technique requires a careful approach to direct the stiff wire; it is best used only in straight vessels as there is a significant risk of perforation. Adequate visualisation of the distal vessel using contra-lateral left coronary injection is imperative. D: Excellent final result following stenting.
Once the cap has been penetrated, particularly in relatively young lesions, the rest of the occlusion may be successfully traversed using a relatively softer wire (which can be exchanged using an over-the-wire balloon or micro-catheter). However, lesion calcification may pose a problem.
Calcification
Relatively recent occlusions have little calcification, and gentle rotation of the wire will enable antegrade progression (Figure 5).
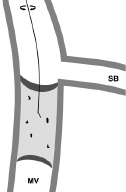
Figure 5. Once the proximal cap has been penetrated, in relatively recent occlusions with little calcification, the lesion may be comparatively easy to cross with gentle rotation (<90˚) of the guidewire.
Heavy calcification however poses a barrier to progression of the wire. Unless a very stiff wire is used, calcium can deflect the wire tip potentially into the sub-intimal space (Figure 6a). Calcification is best evaluated by MSCT rather than angiography, though such scanning is not at present routinely carried out; however, any evidence of calcification could suggest that an initial strategy using a stiff wire might be more effective. If a guidewire does take an incorrect path into the subintima, then when using the parallel wire technique, it might be effective to use a second wire with a stiffer tip in case the problem has been an area of calcification that needs to be penetrated (Figure 6b).
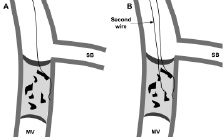
Figure 6A. In lesions with heavy calcification, if the wire tip stiffness < hardness of the calcium, the wire tip will be deflected, in this case into the subintima. B: the first wire is maintained in position thereby blocking entry into the false channel. A second wire is taken in parallel to the first and takes a new (correct) path across the occlusion. In this situation, using a second wire with a stiffer / tapered tip may improve penetration through any areas of calcification.
Microvessels
Neovascularisation within CTOs may have both advantages and disadvantages to percutaneous therapy. When well-developed microvessels run parallel within the occlusion, then this may facilitate passage with a guidewire. Hydrophilic coated wires are useful in this situation as the lubricious coating that is activated when wet, aids the wire to slip through the channel. These microvessels may or may not be visible angiographically, though particularly when occlusions have evidence of antegrade (TIMI I) flow through a central channel, these wires can be very effective and may be used as a first option. Examples include the Crosswire NT (Terumo), the Shinobi wire (Cordis Corporation), and the Whisper and Pilot wires (both from Guidant Corporation). Tapered tip wires may also be able to pass through these microvessels. However, microvessels tend to be friable and when superficial, may increase the risk of perforation with the wire. Bridging collaterals can be deceitful particularly when very tortuous (corkscrew) and angulated, as even though they may appear to be of a reasonable size, wires may frequently perforate through the friable thin wall. In such chronic lesions, it is often better to take a relatively stiff wire and direct it directly through the central path of the occlusion whilst ignoring the superficial collaterals that may run outside of the occlusion.
The distal cap
Penetration through the distal end of the occlusion may also be difficult at times particularly in very long or tortuous occlusions as the torque control of the wire becomes relatively reduced. As described by Dr. Mitsudo, recently, the novel technique of CTO recanalisation via a retrograde approach has been proposed. When passing a lubricious wire through the collaterals into the distal vessel, it has become apparent that the distal end of many occlusions can be crossed in this direction relatively more easily. It is hoped therefore that the use of a combination of antegrade and retrograde approaches may further increase the success rate of CTO recanalisation.
The efficacy of drug-eluting stent implantation
The long-term outcome of CTO therapy is hindered by a relatively high rate of restenosis compared with the treatment of less complex lesions. Several randomised studies5,7,25-31 demonstrated that stent implantation provides superior results when compared with balloon-only angioplasty, but was still associated with restenosis rates of between 22-57%. A recent meta-analysis of these trials has been published;32 this demonstrated no difference in mortality (0.4% after stenting versus 0.7% after balloon-only angioplasty (POBA), (OR 0.72, 95% CI 0.21-2.50). Stenting was associated with significantly less overall MACE (23.2% versus 35.4%, OR 0.49, 95% CI 0.36-0.68), due to significantly fewer repeat revascularisations (17% versus 32%, OR 0.41, 95% CI 0.31-0.53).
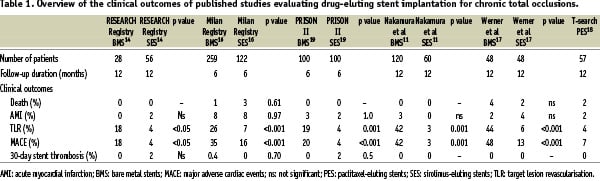
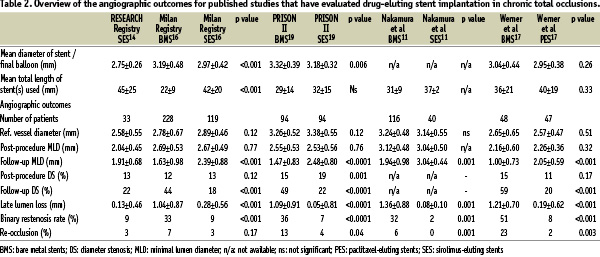
Several registries have published the results of drug-eluting stent (DES) implantation in comparison to a control population treated with bare metal stent (BMS) implantation. Studies have evaluated either the sirolimus-eluting stent (SES, Cordis, Johnson & Johnson), or paclitaxel-eluting stent (PES, Boston Scientific Corp). A summary of the clinical and angiographic outcomes are presented in Tables 1 and 2, and demonstrate significantly fewer adverse events in those treated with DES, with significantly less restenosis and need for target lesion revascularisation (TLR).11,14-18
Importantly, there has been one prospective randomised study (PRISON II) comparing the outcome of the SES compared with BMS implantation in CTOs.19 This confirmed the registry data demonstrating efficacy of the SES through a reduction in TLR (4% for the SES versus 19%, p<0.001). The primary endpoint of the study was angiographic binary in-segment restenosis at 6-months, and was 11% for the SES versus 41% for the BMS (p<0.0001). Taking the results of all of these studies into consideration, the rate of target vessel revascularisation is significantly lower in those treated by DES implantation (Figure 7).
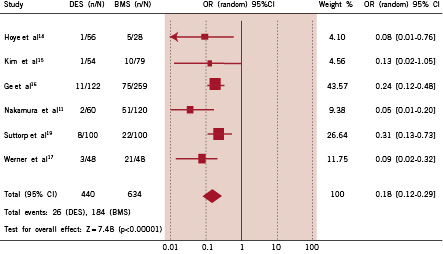
Figure 7. Comparison of the risk of need for repeat revascularisation in patients treated with drug-eluting stent implantation (DES) versus bare metal stent (BMS) implantation in each study and in the overall population, demonstrating odds ratio (OR) and 95% confidence intervals (CI).
Conclusions
Recanalisation of CTOs remains a challenge for interventional cardiologists. We now have specialised wires and an armoury of wiring techniques that have developed over the last 5-10 years. The introduction of these methods enables >80% of CTOs to be successful opened in experienced operator’s hands.33 When looking at an occlusion angiographically, it is important to remember that this is a pretty crude method with relatively poor resolution. Pathophysiology studies have shown that microvessels are frequently present and even “difficult” occlusions may be successfully (and sometimes surprisingly easily) crossed. To improve the success rate of CTO recanalisation, perhaps the most important technique to learn is to take time to cross the lesion in a careful, slow and controlled manner, and become thoroughly familiar with a small group of specialised wires that are used on a regular basis. Once recanalisation has been achieved, drug-eluting stent implantation is associated with an improved subsequent outcome.

