Abstract
Aims: The risk of stent thrombosis has been reported to increase with percutaneous coronary intervention (PCI) complexity. The present study reports the pre-specified secondary endpoint of a 14-month stent thrombosis and major adverse cardiac events in patients stented with a simple versus a complex bifurcation technique using sirolimus eluting stents (SES).
Methods and results: A total of 413 patients with a coronary bifurcation lesion were randomised to a simple treatment strategy; stenting of main vessel and optional stenting of side branch (MV group), or to a complex stenting strategy; stenting of both main vessel and side branch (MV+SB group). Mortality data were available in all patients and 14-month clinical follow-up data in 395 (96%) of the patients. After 14 months, the rates of definite, probable and possible stent thrombosis (ARC criteria) were 1.0% vs. 0.5%, 1.0% vs. 0% and 0.5% vs. 0% (ns) in the MV and in the MV+SB groups, respectively. Rates of MACE were 9.5% in the MV group and 8.2% in the MV+SB group (ns). Total death was seen in 2.4% vs. 1.0% and non-PCI related myocardial infarction in 2.0% vs. 1.0% in the MV and the MV+SB groups, respectively.
Conclusions: After 14 months, two months after recommended cessation of dual antiplatelet therapy, the rates of stent thrombosis and major adverse cardiac events were low and independent of treatment complexity in patients treated with SES for coronary artery bifurcation lesions.
Introduction
Bifurcation lesions are present in about 15% of percutaneous coronary interventions (PCI)1. Treatment of bifurcation lesions used to be associated with increased risk of vessel closure in the acute phase and restenosis on long-term basis. The use of coronary bare metal stents and later drug eluting stents improved the short- and long-term treatment outcome in this complex lesions subset2,3. Thus, the ARTS II study showed similar outcome in sirolimus eluting stent (SES) treated bifurcation and non-bifurcation lesions4. In contrast, other studies have shown increased risk of stent thrombosis in bifurcation lesions, and that the risk of stent thrombosis might be related to the complexity of the bifurcation treatment strategy5,6. In the Nordic Bifurcation study we compared a simple; stenting main vessel and optional stenting of side branch (MV), and a complex treatment strategy; stenting of both the main vessel and the side branch (MV+SB), and documented similar and favourable six months clinical and eight months angiographic outcome in both treatment groups7. The present report on the Nordic Bifurcation Study provides 14-month (two months after the recommended maximal clopidogrel treatment period) follow-up data on stent thrombosis and major adverse cardiac event rates. The purpose was to evaluate the medium term follow-up results and safety of dual antiplatelet therapy discontinuation in patients with a coronary artery bifurcation lesion treated with a simple or a complex stenting strategy.
Methods
Patients and study design
This non-blinded randomised multicentre trial was conducted at 28 cardiology centres in Denmark, Sweden, Finland, Norway and Latvia. From September 2004 to May 2005, a total of 413 patients were enrolled. At 14-month follow-up mortality data were available in 100% and clinical endpoints in 96% (395) of the patients; 199 in the MV and 196 in the MV+SB group. A flow diagram of the study is shown in Figure 1.
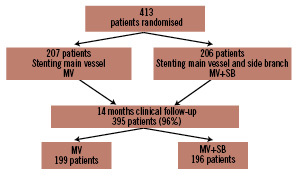
Figure 1. Flow diagram of the Nordic Bifurcation Study.
The protocol was approved by ethics committees in all participating countries, and all participating patients gave written informed consent. The primary results of The Nordic Bifurcation Study as well as the study design have been published7. In short, patients with stable, unstable or silent angina pectoris and a de novo coronary bifurcation lesion defined according to Lefevre et al8, were included. Diameter of main vessel should be > 2.5 mm and of side branch > 2.0 mm by visual estimate.
The sirolimus eluting stent “Cypher Select” (Cordis/Johnson & Johnson, Miami Lakes FL, USA) was used in the study. The study lesion was pre- and/or post-dilated at the discretion of the operator. In the MV group, the main treatment principles were: 1. stenting of main vessel, 2. side branch dilatation if TIMI flow < 3 in the side branch, 3. side branch stenting if TIMI flow = 0 in the side branch after dilatation. In the MV+SB group the main treatment principles were: 1. Stenting of both main vessel and side branch applying the “crush technique”9, “culotte technique”10 or other techniques at the discretion of the operator. 2. In all cases of side branch stenting the operator was required to attempt a “kissing balloon” dilatation at the end of the procedure. The recommended clopidogrel treatment time was 6 to 12 months.
Fourteen month follow-up
Data on total death, cardiac death, myocardial infarction, target lesion revascularisation, target vessel revascularisation and stent thrombosis were obtained by clinical control visit or telephone contact to the patient. National registries and hospital files were used in case of death or admission to hospital for a suspected cardiac event.
Study endpoints
Endpoints were total death, cardiac death, myocardial infarction, target lesion revascularisation (TLR), stent thrombosis and MACE (major adverse cardiac event; cardiac death, myocardial infarction, target vessel revascularisation by PCI or coronary artery bypass surgery and stent thrombosis). The endpoints were adjudicated blindly by an independent endpoint committee chaired by Kristian Thygesen, MD, Aarhus University Hospital, Aarhus, Denmark.
Definitions
Non-procedure related MI: A rise of biochemical markers exceeding the decision limit of myocardial infarction (99th percentile including < 10% CV) with at least one of the following; 1. ischaemic symptoms; 2. ECG changes indicative of ischaemia (ST segment elevation or depression; 3. development of pathologic Q-wave with, no relation to a PCI procedure.
Stent thrombosis (definite, possible and probable) was defined according to the ARC criteria11.
Statistical analysis
Differences in categorical variables between the two groups were analysed using the chi-square test or Fisher’s exact test. Continuous variables were analysed using Mann-Whitney test and time to event data using the Kaplan-Meier method and the log-rank test. All p-values were two-sided. Level of significance was 5%. The analysis was performed on an intention to treat basis. All analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
Funding source
The study coordinators received an unrestricted grant from the Cordis/a Johnson & Johnson Company. The sponsor had no access to the study data and had no role in the design, conduct or reporting of the study.
Statement of responsibility
The authors have full access to the data and take full responsibility for its integrity. All authors have read and agreed on the manuscript as written.
Results
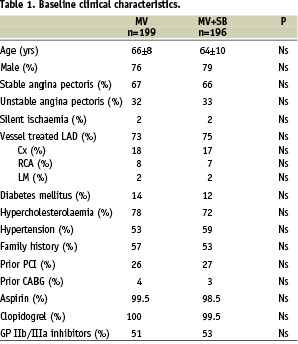
The two groups were well balanced regarding baseline clinical (Table 1) and procedural (Table 2) characteristics.
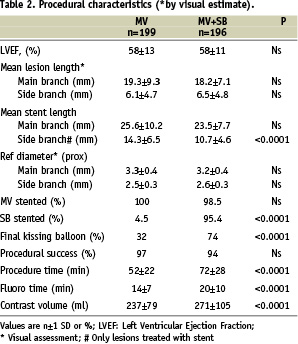
Visual assessed diameter stenosis of >50% in both main vessel and side branch, a genuine bifurcation lesion, was found in 71% of the patients and with similar rates in the two groups. Also, there was no significant difference between the two groups with respect to vessel size or severity of stenosis as assessed by the operator. The lesion characteristics of side branch angulation <70°, vessel calcification and proximal vessel tortuosity were evenly distributed.
At 14 months follow-up, there was no significant difference in the rates of stent thrombosis (Table 3).
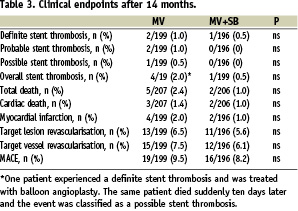
The three patients with definite stent thrombosis after 15, 34 and 35 weeks, were all receiving clopidogrel treatment at the event. The patient with definite stent thrombosis after 34 weeks was treated with balloon angioplasty. The same patient died suddenly ten days later and this event was classified as a possible stent thrombosis.
Two patients died suddenly after four and seven days, respectively (by definition probable stent thrombosis). It is likely that one of these patients stopped clopidogrel at discharge from hospital. Final kissing balloon dilatation was performed in four out of these five patients. The duration of clopidogrel treatment was <6 months in 4.4%, six months in 51.0%, 12 months in 33.5% and >12 months on 11.1%.
Rates of MACE (cardiac death, non-procedure related myocardial infarction, target vessel revascularisation by PCI or coronary artery bypass surgery and/or stent thrombosis) were similar in the two groups (Figure 2, Table 3).
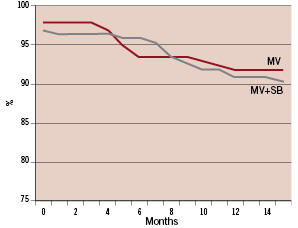
Figure 2. MACE-free survival (cardiac death, non-PCI related myocardial infarction, target vessel revascularisation, stent thrombosis) during 14 month follow-up.
The rates of individual endpoints were low in both groups without significant difference between the groups (Table 3).
Discussion
In the present randomised clinical interventional trial comparing a simple versus a complex bifurcation stenting strategy, we found no significant difference in cardiac death, myocardial infarction, stent thrombosis, target vessel revascularisation or a combination of those at the 14-month follow-up, two months after the maximal recommended clopidogrel treatment time. Moreover, there was no clear relation between discontinuation of clopidogrel and stent thrombosis.
In interventional cardiology, bifurcations represent a challenging coronary lesion subset. Balloon angioplasty was associated with poor result in the acute phase and implantation of bare metal stents with high rates of restenosis, especially if two stents were implanted3. Treatment with drug eluting stents (DES) gave rise to optimistic reports on low rates of acute events and minimal problems with restenosis12,13. Later, a high rate of stent thrombosis was reported in these lesions, and seemed to be associated with treatment complexity. Further, outcome in bifurcation was found to be related to a finalising kissing balloon dilatation14,15.
In the present study, the event rates were low and similar in the complex and in the simple bifurcation treatment group. The rates of overall stent thrombosis were 0.5% in MV+SB group vs. 2.0% in the MV group. Thus, treatment complexity was not associated with increased risk of stent thrombosis, when sirolimus stents were used. Final kissing balloon dilatation was performed twice as frequently (74.0%) in the MV+SB group than in the MV group (31.8%), and might explain the trend to a higher rate of overall stent thrombosis in the MV group.
Roughly, half of the patients stopped clopidogrel after six months, while one third stopped clopidogrel after 12 months treatment. Few patients stopped before three months and about 10% were still on double platelet inhibition at the 14-month follow up. Despite this diversity of clopidogrel treatment time, no clear association with clopidogrel treatment time and stent thrombosis or other adverse events could be detected.
Limitations
This study had an open design; thus operators and patients were aware of the technique used. Primary and secondary endpoints, however, were adjudicated by a blinded event committee and should not be influenced by the open design of the study. The patients studied had a variety of bifurcation lesion types and lesion locations, but a bias towards higher inclusion rates of lesions with small side branches cannot be totally excluded. Therefore, the overall recommendation from the study may not be valid for specific subsets of lesions. Still, 71% of the patients were treated for a genuine bifurcation lesion, i.e., lesions with more than 50% stenosis located both in main vessel and side branch. Finally, a follow-up period of 14 months may be too short to give a definite answer to the possible long term safety problems in SES treatment of coronary bifurcations.
Conclusion
At 14 months follow-up, two months after scheduled discontinuation of double antiplatelet therapy, MACE and stent thrombosis rates were low, indicating an excellent safety profile for both the simple and the complex stenting strategy using SES in coronary bifurcation lesions.
Appendices
The Nordic Bifurcation Study 14-month safety results were presented at the ESC congress, Vienna, September 1-5, 2007.
The Nordic-Baltic PCI Study Group: The purpose of the Nordic-Baltic PCI Study Group is to conduct academic randomised clinical trials and to optimise PCI treatment in the Nordic and Baltic countries.
Steering committee: Leif Thuesen, Aarhus University Hospital, Skejby, Aarhus, Denmark; Jens Flensted Lassen, Aarhus University Hospital, Skejby, Aarhus, Denmark; Jens Aarøe, Aalborg University Hospital, Aalborg, Denmark; Per Thayssen, Odense University Hospital, Odense, Denmark; Henning Kelbæk, Rigshospitalet, Copenhagen, Denmark; Steffen Helqvist, Rigshospitalet, Copenhagen, Denmark; Jan Skov Jensen, Gentofte University Hospital, Gentofte, Denmark; Anders Galløe, Gentofte University Hospital, Gentofte, Denmark; Stefan James, Uppsala University Hospital, Uppsala, Sweden; Iwar Sjögren, Falun Hospital, Falun, Sweden; Terje Steigen, University Hospital of Tromsoe, Tromsoe, Norway; Jan Mannsverk, University Hospital of Tromsoe, Tromsoe, Norway; Oliver Meyerdierks, Ullevaal University Hospital, Oslo, Norway; Pål Gunnes, Feiringklinikken, Feiring, Norway; Svein Rotevatn, Haukeland University Hospital, Bergen, Norway; Rune Wiseth, St. Olav Hospital, Trondheim, Norway; Kjell Nikus, Tampere University Hospital, Tampere, Finland; Saila Vikman, Tampere University Hospital, Tampere, Finland; Juha Hartikainen, Kuopio University Central Hospital, Kuopoi, Finland; Matti Niemelä, Oulu University Hospital, Oulu, Finland; Kari Kervinen, Oulu University Hospital, Oulu, Finland; Kari Virtanen, Helsinki University Central Hospital, Helsinki, Finland; Juhani Airaksinen, Turku University Central Hospital, Turku, Finland; Antti Ylitalo, Satakunta Central Hospital, Pori, Finland; Andrejs Erglis, Paul Stradins Clinical Hospital, Riga, Latvia; Indulis Kumsars, Paul Stradins Clinical Hospital, Riga, Latvia
Participating centres, investigators and inclusions per centre:
Denmark: – Aarhus University Hospital, Skejby, Aarhus; Primary investigator: Leif Thuesen (71 pts.); Jens Flensted Lassen, Jan Ravkilde, Lars Romer Krusell, Hans Erik Bøtker; – Gentofte Hospital Primary investigator: Jan Skov Jensen (31 pts.); Anders Galløe; – Aalborg University Hospital, Primary investigator: Jens Aarøe (16 pts.); – Rigshospitalet, Copenhagen, Primary investigator: Steffen Helqvist (14 pts.); Henning Kelbæk, Erik Jørgensen, Kari Saunamäki; – Odense University Hospital, Primary investigator: Per Thayssen (7 pts.); Knud Nørregaard Hansen
Sweden: – Falun Hospital, Primary investigator: Iwar Sjögren (14 pts.); – Uppsala University Hospital, Primary investigator: Stefan James (7 pts.); – Helsingborg Hospital, Primary investigator: Fredrik Schersten (4 pts.); – Karolinska University Hospital, Huddinge, Primary investigator: Bo Lindvall (3 pts.); – Lund University Hospital, Primary investigator: Göran Olivecrona (3 pts.); – Gävle Hospital, Primary investigator: Lars Hellsten (2 pts.); – Umeå University Hospital, Primary investigator: Lars Lundgren (2 pts.); – Karlskrona Hospital, Primary investigator: Carl-Magnus Pripp (2 pts.); – Södersjukhuset, Stockholm, Primary investigator: Bassem Samad (1 pt.); – Örebro University Hospital, Primary investigator: Thomas Kellerth (1 pt.)
Norway: – Tromsø University Hospital, Primary investigator: Terje Steigen (23 pts.); Jan Mannsverk; – The Feiring Clinic, Oslo, Primary investigator: Pål Gunnes (19 pts.); – Ullevål University Hospital, Oslo, Primary investigator: Oliver Meyerdierks (10 pts.); – Trondheim University Hospital, Primary investigator: Rune Wiseth (7 pts.); – Haukeland University Hospital, Bergen, Primary investigator: Svein Rotevatn (5 pts.); – Rikshospitalet University Hospital, Oslo, Primary investigator: Knut Endresen (3 pts.)
Finland: ; – Oulu University Hospital, Primary investigator: Matti Niemelä (43 pts.); Kari Kervinen; – Tampere University Hospital, Primary investigator: Kjell Nikus (25 pts.); Saila Vikman; – Pori Hospital, Primary investigator:Antti Ylitalo (15 pts.); – Helsinki University Hospital, Primary investigator: Kari Virtanen (7 pts.); – Kuopio University Hospital, Primary investigator: Mikko Puhakka (5 pts.); – Turku University Hospital, Primary investigator: Juhani Airaksinen (5 pts.)
Latvia: – Paul Stradins Clinical Hospital, Riga, Primary investigator: Andrejs Erglis (68 pts.); Indulis Kumsars
Acknowledgements
Coordination and data entry: Hanne Rask Hansen, study secretary, Helle Bargsteen, study secretary, Dorthe Frydensberg, study nurse, Marianne Esbjerg, study nurse.
Data monitoring and safety: Members of the steering committee.
Statistical support: Leif Spange Mortensen, MSc, UNI-C, Danish Information Technology Centre for Education and Research. Helle Højdahl MPH, Aarhus University Hospital, Skejby, Denmark.
Clinical endpoint committee: Kristian Thygesen, MD, Aarhus University Hospital, Aarhus, Denmark. Jan Ravkilde, MD, Aarhus University Hospital, Aalborg, Denmark.

