Abstract
Aims: To describe the procedural performance and 30-day outcomes following implantation using the 18 Fr CoreValve Revalving System (CRS) as part of the multicentre, expanded evaluation registry, 1-year after obtaining CE mark approval.
Methods and results: Patients with symptomatic severe aortic stenosis and logistic Euroscore > 15%, or age > 75 years, or age > 65 years associated with pre-defined risk factors, and for whom a physician proctor and a clinical specialist were in attendance during the implantation and who collected the clinical data, were included. From April 2007, to April 2008, 646 patients with a mean age of 81± 6.6 years, mean aortic valve area 0.6±0.2 cm2, and logistic EuroSCORE of 23.1±13.8% were recruited. After valve implantation, the mean transaortic valve gradient decreased from 49.4±13.9 to 3±2 mmHg. All patients had paravalvular aortic regurgitation < grade 2. The rate of procedural success was 97%. The procedural mortality rate was 1.5%. At 30 days, the all-cause mortality rate (i.e. including procedural) was 8% and the combined rate of death, stroke and myocardial infarction was 9.3%.
Conclusions: The results of this study demonstrate the high rate of procedural success and a low 30-day mortality in a large cohort of high-risk patients undergoing transcatheter aortic valve implantation (TAVI) with the CRS.
Introduction
In 2002, Cribier et al performed the first transcatheter implantation of the aortic valve1. Since then, over 2000 patients have undergone the procedure worldwide by means of two differing technologies. The CoreValve ReValving System® (CRS) and Edwards SAPIEN™ prosthetic heart valve obtained CE (Conformité Européenne) mark approval in April 2007, and August 2007, respectively. Both technologies are currently subjected to post-marketing surveillance studies in Europe.
Recent reports have suggested anecdotally that procedural and 30-day outcomes have improved since the initial safety and feasibility studies2-4. In these early studies, procedural success was achieved in approximately three-quarters of patients and 30-day mortality rate was approximately 15%. Inherent limitations of these initial studies included the initial learning curve, small sample size calculations, use of older generation devices, and represented, for the most part, the experience of single centres.
In view of the staggering increase in the number of transcatheter aortic valve implantations performed worldwide, and the inherent limitations of the initial safety and feasibility studies, we report on the procedural success and outcomes at 30 days, in 646 patients with symptomatic severe aortic stenosis, who underwent implantation with the third generation (18F) CRS during the first year of the multicenter, expanded evaluation registry.
Methods
Between April 23, 2007 and April 23, 2008, 729 patients with symptomatic, aortic valve stenosis who were considered high risk or non-surgical candidates underwent implantation with the third generation 18 Fr CRS as part of the multicentre expanded evaluation registry. This report examined the outcomes of the 646 patients for whom a physician proctor and a clinical specialist were in attendance during the procedure and who collected the clinical data (i.e. baseline characteristics and procedural data) prospectively. As such, these patients represented “learning curve” cases. A clinical specialist was not present for 83 (11.4%) of the implant procedures, and therefore, a collection report form was not completed for these cases. Indeed, these cases were performed in centers with greater clinical experience. Although a physician proctor was scrubbed for each case, their primary role was to advise and supervise the physician performing the procedure. A total of 51 centres participated in the registry (see appendix 1 for number of implantations per country).
Inclusion criteria included the following: (1) aortic valve area < 1 cm2 (<0.6 cm2/m2); (2) aortic valve annulus diameter measuring > 20 mm and < 27 mm; (3) sinutubular junction measuring > 43 mm, determined by echocardiography; (4) diameter of the femoral vessel > 6 mm. In addition, patients had to be (5a) > 75 years of age or (5b) have a predicted surgical mortality rate by logistic EuroSCORE > 15% or (5c) age > 65 years and present with one or more of the following complicating factors – cirrhosis of the liver (Child class A or B), pulmonary insufficiency defined as a forced expiratory volume in one second (FEV1) < 1 litre, pulmonary hypertension (i.e. pulmonary artery systolic pressure > 60 mmHg), previous cardiac surgery (coronary artery bypass graft surgery or valvular surgery), porcelain aorta, recurrent pulmonary emboli, right ventricular insufficiency, contraindication to open chest surgery (i.e. previous chest burns or radiation), or cachexia (body mass index less than or equal to 18 kg/m2).
Exclusion criteria comprised any of the following: known hypersensitivity or contraindication to antiplatelet or anticoagulant therapy, nitinol, or contrast media that could not be adequately pre-medicated; any sepsis; myocardial infarction in the preceding 30 days; percutaneous coronary intervention within the 15 days prior to implantation or scheduled during or within 30 days after transcatheter valve implantation; any left atrial or ventricular thrombus; left ventricular ejection fraction < 20%; uncontrolled atrial fibrillation (heart rate greater than 100 beats per minute); mitral or tricuspid valvular insufficiency greater than grade 2; history of aortic valve replacement; any condition considered as a contraindication for extracorporeal assistance; recent stroke; severe peripheral arterial disease that make insertion and endovascular access to the aortic valve impossible; symptomatic carotid or vertebral artery disease (i.e. > 70% stenosis); abdominal aortic aneurysm; bleeding diathesis or coagulopathy; creatinine clearance < 20 ml/min; and a life expectancy of less than one year.
A table describing additional selection criteria based on imaging characteristics (angiography, echocardiography, multi-slice CT, and magnetic resonance imaging) of the aortic valvar complex and left ventricle is included in appendix 2.
A patient was considered high risk if there was consensus among the cardiovascular team (i.e. cardiologist and cardiac surgeon) that conventional open-heart surgery would be associated with excessive morbidity and mortality. All patients provided written consent prior to the procedure.
Device description and procedure
Details of the device, and the technical aspects of the procedure, have been published previously3,5. Briefly, the CRS consists of the delivery catheter system, a disposable compression and loading system and a percutaneous aortic valve bioprosthesis (Figure 1).
A
B
C

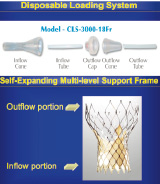
Figure 1. Illustration of the components of the CoreValve ReValving System: (A) Delivery catheter system, (B) Disposable loading system, (C) Percutaneous self-expanding aortic valve bioprothesis.
The percutaneous aortic valve consists of a self-expanding, nitinol tri-level frame to which is sewn a single layer of porcine pericardium built in a trifoliate configuration. A scalloped skirt that is also made of a single layer of porcine pericardium is attached to the inflow portion of the valve prosthesis. Together with the radial force imparted by the inflow portion of the valve, the skirt attempts to create a seal and mitigate paravalvular aortic regurgitation. Currently, the prosthesis is available in sizes of 26 and 29 mm inflow diameter for patient annulus diameters between 20 and 27 mm. Selection of the size of the prosthesis depends on measurements of the aortic valvar complex obtained by echocardiography, aortography, or multi-slice computed tomography. The delivery catheter system is 18 Fr at its distal end (i.e. deployment end) and 12 Fr at its proximal end (i.e. shaft component).
Depending on the preference of the physician and the medical condition of the patient, the procedure was performed with the patient under general anaesthesia or local anaesthesia and mild sedation. Vascular access was performed in a completely percutaneous fashion with the aid of a vascular “pre-closing” device (10 Fr Prostar XL). The recommended pre-medication regimen consisted of aspirin 80 mg once daily and clopidogrel 75 mg once daily each for three days prior to the procedure or a loading dose of clopidogrel 300 mg on the day of the procedure. If the patient was unable to tolerate clopidogrel, it was substituted with ticlopidine 250 mg twice daily, each day for three days prior to the procedure or a loading dose of 500 mg the day of the procedure. Furthermore, one hour prior to the procedure, prophylactic antibiotic therapy was administered according to local practice guidelines. During the procedure, heparin, weight adjusted, was given with a goal of obtaining an activated clotting time (ACT) of approximately 250-300 seconds for the duration of the procedure. Post implantation, a dual antiplatelet strategy of aspirin 80 mg and plavix 75 mg, each daily, for 6 months, followed by aspirin 80 mg indefinitely was prescribed.
Definition of endpoints
Procedural success was defined as adequate technical placement of the device within the aortic root, adequate functionality of the device immediately after implantation, and the event where the patient leaves the catheterisation laboratory alive. “Adequate technical placement of the device within the aortic root” evaluated the system’s ability to (1) load the valve into the delivery catheter; (2) access the vasculature; (3) cross the arch of the aorta (4) access the aortic valve with the delivery catheter; (5) deploy the valve accurately across the aortic native valve annulus and; (6) remove the intact delivery catheter system. “Functionality of the device” was defined by a reduction in the mean trans-aortic valve gradient to less than 20 mmHg and aortic regurgitation grade < 2 as assessed by invasive haemodynamic methods implementing fluid-filled catheters and contrast aortography or echocardiography. Of note, a patient could have experienced a complication during the procedure (e.g. left ventricular perforation) and still have satisfied the requirements for procedural success.
Procedural outcome events represented those events occurring during the procedure and within the subsequent 24 hours. In particular, procedural-related death, cardiovascular death, neurological events (i.e. transient ischaemic attacks and stroke), myocardial infarction, and complications related to the procedure (coronary artery flow impairment as a result of the prosthesis implantation, aortic root dissection, left or right ventricular perforation, cardiac tamponade, vascular access site bleeding, urgent conversion to conventional open-chest surgery) were documented. Procedural-related death was defined as any death that was adjudicated to be a result of an intra-procedural complication.
Thirty-day outcome events included procedural outcome events and those occurring through 30 days. In particular, death from all cause, cardiovascular death, procedural-related death, myocardial infarction, neurological events (i.e. reversible transient ischaemic attacks and irreversible stroke), and the need for permanent pacemaking were documented.
Post-procedural valve performance (i.e. mean transaortic valve gradient, and valve regurgitation) was assessed by either invasive haemodynamic methods or transthoracic echocardiography prior to discharge.
Statistical analysis
Continuous variables are presented as means±standard deviation (SD). Categorical variables are presented as frequencies and percentages. When applicable, 95% confidence intervals are provided. Events are reported in a non-hierarchical fashion.
Results
A flow diagram of the patients recruited for analysis is shown in Figure 2.
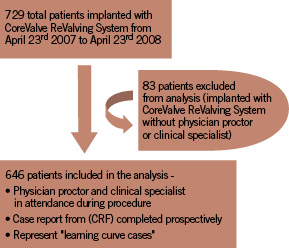
Figure 2. Flow diagram of patients included for analysis of registry results.
The baseline characteristics of the patients are summarised in Table 1.
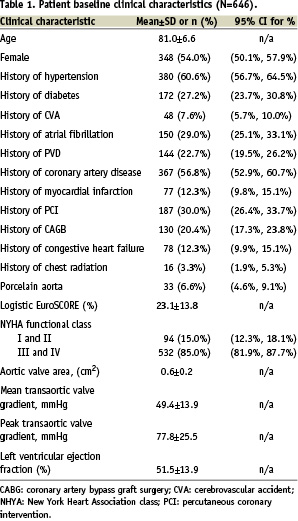
The mean age of the patients was 81±7 years and 54% were female. Prior to the procedure, the mean transaortic valve gradient was 49.4±13.9 mmHg and mean effective aortic valve orifice area of 0.6±0.2 cm2. Eighty-five percent of the patients were diagnosed with New York Heart Association class III or IV. The mean logistic EuroSCORE was 23.1±13.8%.
Procedural-related outcomes
Clinical
Procedural success was achieved in 97% of the patients (Table 2).
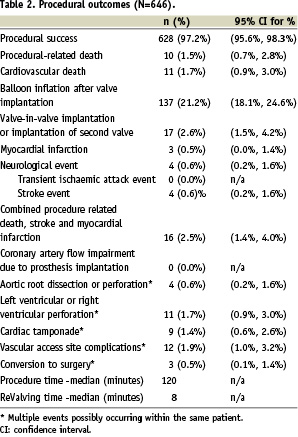
Procedural death occurred in 1.5% of the patients. The combined incidence of procedural death, myocardial infarction, or stroke was 2.5%.
Vascular access site complications (dissection or tear) were reported in 12 out of 646 patients (1.9%) (Table 2). More specifically, four of these patients [0.6%, 95% confidence interval for percentage (0.2%; 1.6%)] experienced a retroperitoneal haemorrhage.
Technical considerations
There were no cases of the device obstructing or jailing the coronary arteries. Balloon inflation of the annulus of the frame after implantation of the device was performed in 137 out of 646 (21.2%) of procedures to improve seating in the space of the annulus and to mitigate moderate to severe aortic regurgitation. A second valve was implanted in 2.6% of patients.
The median total duration of the procedure was 2 hours, and the median implantation time of the prosthesis i.e. ReValving time, was 8 minutes.
Outcomes at 30 days
At 30 days, death from all-cause was 8% (Table 3).
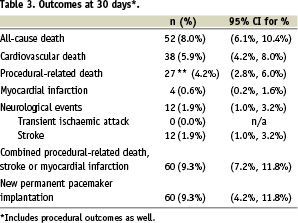
One-half of these deaths were judged to be procedural-related although this finding included three patient deaths where the cause of death was uncertain. Cardiovascular causes accounted for approximately three-quarters (38/52) of the deaths.
There were eight stroke events that occurred more than 24 hours after the procedure. Four out of the eight occurred two days after the procedure date, and a delayed stroke event occurred at three, five, seven and nine days after the procedure.
Permanent pacemaking was needed in 9.3% of patients.
Post-procedural prosthetic valve performance
Transthoracic echocardiography performed prior to discharge demonstrated a significant reduction in transaortic valve gradients (50.4±16.8 vs. 3.2±5.2 mmHg, pre-treatment vs. post-treatment). Following implantation, 100% of patients had < grade 2 aortic regurgitation. Moderate to severe aortic regurgitation diagnosed during the index procedure was managed with balloon re-dilatation and/or implantation of a second valve.
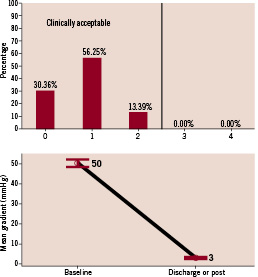
Figure 3. Post-procedural prosthetic aortic valve performance. (Top) Aortic regurgitation severity post-implantation. (Bottom) Invasive measurements of mean transaortic valve gradient (mm Hg) pre- and post-implantation.
Discussion
This is the largest report investigating the outcomes of patients undergoing transcatheter implantation of the aortic valve. The procedural success rate was 97% and the 30-day all-cause mortality rate was 8% in a cohort of 646 patients who underwent implantation with the 18 Fr CRS during the first year of the multicentre, expanded evaluation registry.
The rate of procedural success reported in the initial safety and efficacy studies were very encouraging. Cribier et al reported a procedural success rate in four-fifths of patients who received the Cribier-Edwards prosthetic heart valve2. However, 26 patients underwent implantation by an antegrade approach and only seven patients by a retrograde approach. For various reasons, the latter has supplanted the former approach, and therefore, any meaningful comparison to the results of more recent reports would appear unjustified. In a report of 86 patients undergoing implantation with the CRS, Grube et al reported procedural success rates of 78% and 69% for the 21 Fr and 18 Fr CRS, respectively3. Furthermore, Webb et al demonstrated an overall procedural success rate of 86% in their first 50 patients implanted with the Edwards SAPIEN prosthetic heart valve4.
In this registry study, the procedural and 30 day mortality rates were 1.5% and 8%, respectively. In the report by Grube et al, the procedural and 30-day mortality rates were 6% and 12%, respectively3. Webb et al documented a procedural mortality rate of only 2% and similar 30-day mortality rates4.
The 30-day mortality rate documented in this report also compares favorably to contemporary surgical reports of high-risk patients undergoing replacement of the aortic valve. In particular, octogenarians undergoing surgical replacement of the aortic valve have a 30-day mortality rate of approximately 6.1-9.3%6-9. Furthermore, patients with documented dysfunction of the left ventricle and a low mean transaortic valve gradient have a 30-day surgical mortality rate ranging between 8-33%, influenced greatly by the presence or absence of contractile reserve10-12.
It is noteworthy that 4 stroke events were diagnosed within 24 hours of the procedure and 8 “delayed” stroke events were diagnosed 24 hours after the procedure but all within 9 days of the index procedure. Although the reason for the diagnosis of these “delayed” stroke events is not clear, a time-lag from the onset of symptoms to the diagnosis of the neurological deficit and interpretation of the radiographic imaging may be an important factor. This observation may also warrant a reconsideration of the antiplatelet or antithrombotic regimens that are recommended currently.
In this study, the need for permanent pacemaking was documented in approximately one tenth of patients and represented the most common event occurring within 30 days of implantation of the device. In a recent, single centre study of 40 patients who underwent implantation with the CRS, a permanent pacemaker was implanted in 18% of patients13. Furthermore, in this study, the distance from the lower edge of the non-coronary aortic cusp to the inflow edge (i.e. ventricular end) of the frame of the prosthesis was measured to be a mean of 10.3 mm (SD±2.7 and range 6.7 to 14.6 mm) in those with left bundle branch block of new onset distinctively after valve implantation, and 5.5 mm (SD±3.4 and range 0.7 to 12.2 mm) in those without the development of left bundle branch block (p=0.005). These results seem to suggest that the occurrence of conduction abnormalities may be related to the depth of implantation of the prosthesis within the left ventricular outflow tract, and that, a more superior positioning of the prosthesis within the left ventricular outflow tract might possibly decrease the incidence of conduction abnormalities and need for pacemaking. In another recent, single centre study of 123 patients implanted with the balloon-expandable Edwards SAPIEN prosthetic heart valve, 5.7% of patients developed a new and sustained complete AV bock requiring pacemaker implantation14. Similarly, the need for permanent pacemaking in elderly patients undergoing surgical aortic valve replacement has been reported to be 6.0-6.5%15-18. Indications for implantation of a permanent pacemaker following valve implantation should follow the ACC/AHA and ESC guidelines19,20. It should be noted, however, that permanent pacemakers are occasionally implanted on a “prophylactic” basis following valve implantation. This may occur, for example, in the context of asymptomatic bradycardia or the occurrence of left bundle branch block after valve implantation. The latter practice stems from literature suggesting that new and persistent left bundle branch block acquired after surgical aortic valve replacement has been associated with significantly increased risk of subsequent arrhythmic events, such as syncope, atrioventricular dissociation, and sudden cardiac death21. Whether such practice is warranted after transcatheter valve implantation is not known and further study is required.
The results of this study confirm the significant reduction in mean valve gradient after transcatheter aortic valve implantation2-4. Although 70% of the patients had grade 1 or 2 paravalvular aortic regurgitation, previous reports following surgical aortic valve replacement have also estimated that paravalvular leaks can be detected in up to three-fourths of patients, are usually small and have a benign clinical course22-24. In addition, approximately one-fifth of patients required balloon inflation after valve implantation to improve seating of the prosthesis in the space of the annulus and mitigate grade 3 or 4 aortic regurgitation. Up until now, acute adverse effects to valve functioning due to balloon re-dilatation have not been noted.
Since the initial safety and feasibility studies, there has been a remarkable intellectual and technical learning curve. Presently, there is a better understanding of the process of selection of patients prior to transcatheter valve implantation and postoperative monitoring and management. With increasing experience, Webb et al demonstrated procedural success to increase from 76% in the first 25 patients to 96% in the second 25 patients and an associated decrease in 30-day mortality from 16% to 8%, respectively4. Furthermore, the devices themselves have undergone a number of technological improvements that have undoubtedly led to an improvement in patient outcomes. Among others, these have included alterations in the height of the skirt of the prosthesis that mitigate paravalvular aortic regurgitation, reductions in the profile of delivery catheters, and changes to the delivery catheter systems that facilitate navigation across the aorta and native aortic valve.
The scope of post-marketing surveillance
While clinical evidence is a cornerstone of the pre-market conformity assessment process, it is important to recognise the limitations inherent to these pre-market clinical investigations. For instance, the relatively short follow-up periods and small patient numbers in these pre-market investigations does not allow the manufacturer to detect infrequent complications or those associated with longer-term follow up. Thus, as part of the manufacturer’s quality control process, a program of post-marketing surveillance is essential in identifying and investigating the risks with the use of the device after being placed on the market.
There are a number of strategies that may be used for post-marketing surveillance. For example, these may include complaint handling and vigilance, active supervision by customer surveys, inquiries of users and patients, literature reviews, and post-marketing clinical follow-up. In particular, post-marketing clinical follow-up should always be considered for devices where identification of possible emerging risks and the evaluation of long-term safety and performance are critical. Since the latter points are inevitably relevant to novel heart valve substitutes, post-marketing clinical follow-up is indispensable for these implants25. The aims of post-marketing clinical follow-up are summarised in Table 4.
Post-marketing clinical follow-up may take the form of an extended follow-up of patients enrolled in pre-market trials, a prospective study of a subset of patients after the device is placed on the market, and/or an open registry.
Limitations
As with any analysis of registry data, our study has its inherent limitations. Although dedicated clinical specialists were responsible for the collection of data prospectively, there may have been, nonetheless, missing data for patient baseline characteristics, and inconsistencies and under-reporting of adverse events. In particular, the reporting of data was less than 90% complete for certain patient baseline characteristics such as history of atrial fibrillation, history of chest radiation, history of porcelain aorta, aortic valve area, mean and peak transaortic valve gradient. As a result of insufficient patient information, the estimated effective aortic valve orifice area following the procedure was not reported. The current analysis focused on outcome events collected up to 30 days and this study therefore, was unable to detect complications associated with longer-term follow-up. Furthermore, a Core Lab was not involved in the collection and/or analysis of the clinical outcome data.
Acknowledgements
We would like to acknowledge MedPass International (Paris, France) and Dr. Susan Ludgate for their support in our discussion of post-marketing surveillance.
Appendix 1
Number of CoreValve ReValving System implantations per country (n=729): Austria (49), Belgium/Luxembourg (13), Brazil (2), Columbia (4), Denmark (20), France (29), Germany (315), Italy (89), Netherlands (63), Norway (10), Portugal (7), Spain (9), Sweden (8), Switzerland (29), United Kingdom (82).
Appendix 2
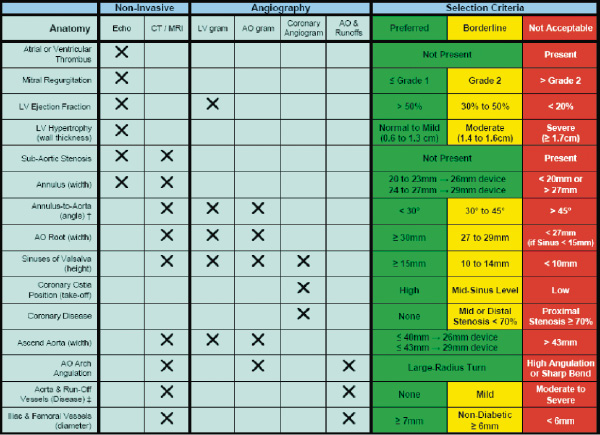
† Within the first 7 cm of the ascending aorta versus a perpendicular line across the aortic valve.
‡ Evaluate for evidence and degree of calcification, obstruction, tortuousity, and ulceration.

