Abstract
Aims: To conduct a clinical registry of consecutive “real world” patients treated using the PRO-Kinetic stent at Rabin Medical Centre between January 2006 and March 2008.
Methods and results: Our study included 515 consecutive patients treated for 540 lesions. We had a 100% follow-up rate at six months. The patients’ characteristics are noted as having a 43% rate of diabetics and a 17% renal insufficiency. Indications for angioplasty included patients with either stable or unstable clinical scenarios and 60% of the lesions were of class B2/C. The mean RVD was 2.81 mm and lesions length was 12.6 mm prior to PCI and with angiographic success in 99.8% of treated lesions. The six month clinical outcome data were as follow: MACE rate of 8.7% with 3.5% total mortality, 1.9% cardiac mortality, 1.4% of any MI and TVR rate of 6.4% per treated patient and 6.7% per treated lesion and 0.4% rate of stent thrombosis.
Conclusions: Based on our findings, 1) The PRO-Kinetic clinical data shows excellent results on a large group of coronary patients, treated consecutively. 2) The data should emphasise the remaining role of ‘state of the art’ non DES platforms (i.e. such as PRO-Kinetic) for patients with a wide variety of characteristics and/or clinical syndromes.
Abbreviations and acronyms
BMS: bare metal stent
DES: drug eluting stent
CABG: coronary artery bypass surgery
LVEF: left ventricular ejection fraction
MLD: minimal lumen diameter
MACE: major adverse cardiac events
PCI: percutaneous coronary revascularisation
QCA: quantitative coronary angiography
TVR: target vessel revascularisation
TLR: target lesion revascularisation
Introduction
The performance of bare metal stents has vastly improved in recent years. Improvements in three-dimensional geometrical structure and the use of thinner metals and innovative delivery systems have enhanced the flexibility and tracking properties (i.e. manoeuvrability) while optimising the scaffolding of the atherosclerotic plaque1. These properties may translate into improved long-term clinical outcomes. The search for a better bare metal stent (BMS) design are of fundamental importance for further definition of patients that are still eligible for BMS utilisation in the current era. The PRO-Kinetic stent (Biotronik Corp., Bülach, Switzerland) is a new generation BMS that combines the properties of the cobalt chromium metal alloy with thin-struts design to allow improved stent performances in all procedures.
The primary aim of this single-centre, non-randomised, consecutive registry was to evaluate the short and intermediate-term clinical performance of the PRO-Kinetic coronary stent in the treatment of patients enrolled in every day practice at our institution.
Methods
Patient population
The current study includes 515 patients who were treated at our institution between January 2006 and March 2008. Patients who were at least 21 year of age, had stable or unstable angina or myocardial ischaemia or acute/recent myocardial infarction, and were undergoing percutaneous coronary intervention (PCI) with bare metal stents were considered for the study. Patients were excluded if they had known allergy to aspirin, clopidogrel, ticlopidine, heparin, or other anticoagulant or anti-platelet therapy as required for PCI. During the study period, the use of drug eluting stents (DES) averaged ±40% at our catheterisation laboratories. This pattern of DES / BMS use is based on guidelines adopted by the Israeli Society of Cardiology (e.g. recommended DES use primarily in LAD lesions, proximal segments, long [>20 mm] and in stent restenosis lesions and/or diabetic patients). Those DES treated patients were obviously excluded from the current analysis. Only patients with ‘pure’ PRO-Kinetic utilisation (i.e. no mix of PRO-Kinetic stent with other BMS or DES stents) were included in the analysis. Written informed consent was obtained from all patients prior to PCI. Data collection was approved by the hospital ethical committee.
Coronary angiography and intervention were performed according to the standard clinical practice at the investigation sites. Dual anti-platelet therapy with clopidogrel (75 mg once daily), supplemented with 100 mg aspirin was recommended for three months for stable patients and for 6-12 months following acute coronary syndrome events. Aspirin alone was recommended indefinitely afterwards (unless contraindicated). Administration of GP IIb/IIIa inhibitors or bivalirudin (instead of heparin ± GP IIb/IIIa) was optional and was left to the investigators’ discretion.
The PRO-Kinetic™ stent system
The PRO-Kinetic™ stent system is a cobalt chromium alloy tubular stent with a unique multi-cellular design, pre-mounted on a semi-compliant, low profile balloon (Figure 1).

Figure 1. The Pro-Kinetic stent.
The cell structure provides improved flexibility and tracking properties in tortuous vessels while radial strength and vessel bend coverage are maintained by the tubular design. The PRO-Kinetic stent has thin struts (60 µm/0.0024” per 2.0-3.0 mm stent, 80 µm/0.0031” per 3.5-4.0 mm stent, and 120 µm/0.0047” per 4.5-5.0 mm stent), a low profile, high deliverability and a silicon carbide PROBIO® passive coating which may allow properties of utilisation during all PCI procedures. The PROBIO coating was shown in vitro to reduce the thrombogenic properties of metal stents, while facilitates regenerative endothelial coverage2.
Endpoints of the registry
The primary endpoint of this registry was a composite of major adverse cardiac events (MACE, for definition see below) at one and six months after the procedure. Secondary endpoints were angiographic and procedural success rates, TLR and TVR rates at six months and angiographically proven in-stent thrombosis at six months. A pre-specified sub-analysis was performed for diabetic patients.
Definitions
Angiographic success was defined as final residual stenosis of the target site <30% using the assigned treatment device alone. Procedural success was defined as angiographic success without in-hospital major adverse cardiac events (MACE). MACE is composed of any death, any myocardial infarction (Q and non Q MI), or any percutaneous or surgical target vessel revascularisation (TVR) or target lesion revascularisation (TLR) procedure. Deaths were classified as cardiac or non-cardiac. Deaths from undetermined causes were reported as cardiac. Myocardial infarction was diagnosed on the basis of the development of new Q waves of more than 0.04 seconds in two or more contiguous leads, accompanied by a significant increase in the creatine kinase or MB isoform or troponin I or T levels; non-Q wave infarction was diagnosed as an increase in the creatine kinase level to more than three times the upper limit of the normal range with an accompanying elevation in the MB isoform or troponin I or T levels without development of new Q waves. Stent thrombosis was classified as acute when it occurred within 24 hours of the index procedure, sub-acute when it occurred between one and 30 days, and late when it occurred beyond 30 days. Stent thrombosis was considered ‘definite’ when confirmed angiographically. Stent thrombosis was considered ‘probable’ when the patient had a target vessel–related MI or died of a coronary event, possibly caused by stent thrombosis, within 30 days of the index procedure. All reported re-interventions inside the stent implanted during the index procedure or within 5 mm proximal or distal to the stent were classified as TLR. Other repeated PCI in the same vessel were recorded as TVR. Renal insufficiency was defined according to serum creatinine values of ≥1.5 mg/dl during hospitalisation. Left ventricular function was diagnosed by either ventriculography or using cardiac echocardiography during hospitalisation. The cardiac function measure was available in 380/515 (77%) of treated patients. Two senior cardiologists and an experienced research coordinator adjudicated all events.
Angiogram assessment
Quantitative coronary angiograms obtained before and after the procedure were analysed at our core laboratory (Rabin Medical Centre, Petach Tikva, Israel) by the Medcon-McKesson Horizon™ system (Tel Aviv, Israel). Baseline angiographic variables included reference vessel diameter (RVD), minimal luminal diameter (MLD), percent diameter stenosis, lesion length and lesion complexity as defined by the American Heart Association/American College of Cardiology (AHA/ACC) classification. Type B2 and C were considered complex lesions. In-stent diameter stenosis, RVD, MLD, acute gain (final MLD-pre MLD) and the presence of dissection were noted post-procedure. No systematic angiographic follow-up was scheduled for this study. However, all performed repeat angiographic procedures were analysed by the same core laboratory. Thrombolysis in myocardial infarction (TIMI) flow grade (0 to 3) was measured prior to and at completion of procedure.
Follow-up
All patients underwent a clinical follow-up for six months. They were scheduled to have a six-month telephone contact. Study patients were enrolled in two hospitals (Beilinson Hospital and Hasharon Hospital, both belong to the Rabin Medical Centre). Telephone contact rates and source document adjudication was 100% at six month time points. At the time of follow-up contact, data were collected pertaining to current clinical status, prior hospitalisation and occurrence of any of the aforementioned adverse events.
Statistical plan
Statistical analysis was done by using STATISTICA™ software in order to calculate the categorical demographic and clinical outcome variables. MACE rates were calculated as hierarchical in case of multiple events (e.g. MI, TVR or death) occurring in individual patients. TVR and TLR events were calculated twice: per patient and per treated vessel, accordingly.
Results
Patient’s demographics
The analysis includes 515 patients treated for 540 coronary lesions using the PRO-Kinetic stent alone. Patient’s demographics are given in Table 1.
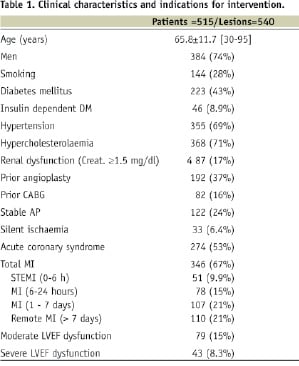
Of note, 223 (43%) patients were diagnosed as diabetics and 46 patients (8.9%) needed insulin treatment. Also, 87 (17%) of the treated patients had some degree of renal insufficiency defined as creatinine ≥1.5 mg/dl. Otherwise, patients’ demographics are representative of a contemporary PCI practice. Clinical indications for PCI are presented as well in Table 1. Of note, acute coronary syndrome event was the cause in about half of the patients. Fifty-one (9.9%) were treated during the course of ST elevation acute myocardial infarction (STEMI) and additional 78 (15%) patients sustained a recent infarction event (STEMI or non-STEMI) within six to 24 hours prior to undergoing PCI. Overall, the majority of patients (67%) had prior MI, either acute or recent or “remote” (>7 days before PCI) event. Accordingly, 79 patients (15%) had moderate LV dysfunction and 43 (8.3%) patients had severe LV dysfunction defined by either left ventriculogram or echocardiographic examination performed during the index hospitalisation.
Lesion characteristics
QCA data were available for all 540 lesions (100%). Main lesion characteristics are presented on Table 2.
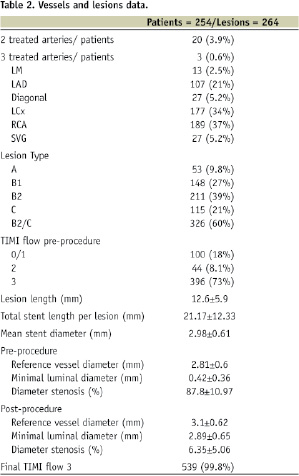
The vast majority of patients (95%) underwent a single vessel intervention. Almost two-third of lesions were categorised as B2 and C type. The left anterior descending artery (LAD) was treated in only 21% of patients as a predominant proportion of LAD diseased patients were treated using DES at our institution. Due to a similar reason, there were only 4% of the stented lesions that involved a true bifurcation lesion. Prior to intervention, the TIMI flow was 3 in only 73% of patients as the remaining patients (27%) sustained acute or recent MI and manifested sub-optimal flow (i.e. TIMI 0-2) into the culprit vessel. The mean reference vessel diameter was 2.81 mm and the average lesions length was 12.6 mm (range 3 to 62 mm) according to QCA data. The total stent length and diameter was 21.2 and 2.98 mm, respectively (Table 2). The final TIMI flow was 3 in 99.8% of the treated vessels as was the rate of angiographic success.
Clinical follow-up
During six month follow up (Table 3), the cumulative MACE rate was 8.7%.
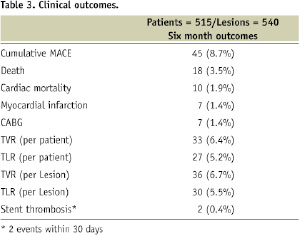
Mortality rate was 3.5% and cardiac mortality rate was verified in 1.9% of patients. Seven patients (1.4%) sustained MI during follow-up, the same number of patients needed coronary bypass surgery (1.4%) and TVR and TLR rates (calculated per treated patient) were 6.4% and 5.2%, respectively. Importantly, there were only two cases (0.4%) of confirmed early or sub-acute stent thrombosis and not a single case of later (>30 days) definite thrombosis event. A graphic summary of the study main outcomes is shown in Figure 2.
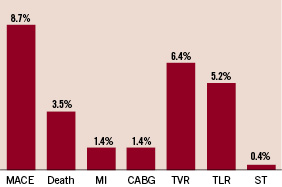
Figure 2. Cumulative (hierarchical) six months results of the study cohort.
Diabetics and non-diabetics subgroup analysis
The MACE rate was 10.7% among the 224 diabetic patients (233 lesions) that participated in the registry. Total mortality was 4.5%, MI rate was 2.7% and TVR and TLR rates (calculated per treated patient) were 7.6% and 7.1%, respectively (Figure 3).
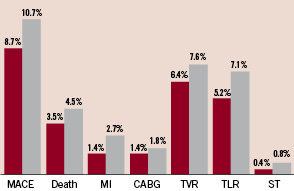
Figure 3. Cumulative (hierarchical) six months results of the total study population (red bars) and the diabetic sub-group (grey bars).
The corresponding figures were lower among the non-diabetic patients with an overall MACE rate of 5.8% during six months follow-up.
Discussion
The main findings of our study are as follow: 1) the PRO-Kinetic clinical data shows very good results on a large group of coronary patients treated consecutively, 2) very few stent thrombosis events (0.4%) were observed and/or confirmed up to six months, 3) the TVR/TLR rates were very low overall and acceptable even among diabetic patients, 4) in a public system and clinical setting, with ~40% of patients treated using DES and 60% of the remaining patients receiving BMS, the data should emphasise the encouraging safety and clinical performance of the PRO-Kinetic coronary stent system among patients with a wide variety of clinical characteristics and syndromes. Additional findings of our study are related to the excellent angiographic performance (99.8% angiographic success rate) and clinical safety and efficacy profiles of the PRO-Kinetic stent in the intermediate-term follow-up period (i.e. MACE rates at six months was 8.7%). Thus, the PRO-Kinetic registry emphasises the need for ongoing discussion concerning the role of contemporary non-DES platforms, in the modern interventional cardiology practice. Also, our results reassure the important role of BMS utilisation in patients faced with the hazards of receiving long-term clopidogrel treatment following DES due to advanced age, bleeding risks, planned surgery, or if they are on concurrent warfarin treatment3.
Comparative data
Our results are in accordance with a previous report by Dahm et al4. They recently reported on 145 patients and 161 lesions treated using the PRO-Kinetic stent. Diabetic patients were 28% of the study population and the mean reference vessel diameter was 2.67 mm with an average lesion length of 12.8 mm. The six month TLR rate was 4.9%, the overall MACE rate was 5.6% and the average angiographic late loss was 0.75 mm. Interesting, an in vitro sub-study showed a favourable and rapid rate of endothelisation associated with the silicon carbide coating as compared to an uncoated stent platform. Thus, it is possible that the PROBIO silicon carbide passive stent coating contributed to the favourable clinical results in the PRO-Kinetic studies2,4.
It is difficult to compare our registry results with other historical stent registries. Nonetheless, we should focus on some of the most recent ones. The MATSURI multicentre registry using the TSUNAMI stainless steel stent, had 1,437 patients and 1,792 lesions with 25% diabetics, 49% type B2/C lesions and 39% located in the LAD5. Lesions length was 10.6 mm and the mean reference vessel diameter was 2.96 mm. The cumulative MACE rate at six months was 7.3% and a TLR rate of 4.5% and 0.2% suspected rate of stent thrombosis.
The Driver cobalt chromium US stent registry6 had 298 coronary patients and lesions (28% diabetics), 45% LAD lesion and 51% type B2/C lesions, with a mean reference vessel diameter of 3.07 mm and a mean lesion length of 11.04 mm. The cumulative MACE was 5.7% and TLR was 3.4% at six months and no sub-acute stent thromboses events were observed. The Multi-Link Vision cobalt chromium coronary US stent registry7 had 267 coronary patients and lesions (23% diabetics), 39% LAD and 40% type B2/C lesions, with a mean reference vessel diameter of 2.94 mm and a mean lesion length averaging 10.6 mm. The cumulative incidence of MACE was 6.2%, TLR was 4.3% and TVR was 5.1% at six months. The mean in-stent late loss was 0.83 mm and no sub-acute stent thrombosis events were reported. The multicentre NIRtop study examined the results of the stainless-steel NIRflex stent in 305 patients suffering from a native coronary disease8. One hundred and fifty-eight (n=158) patients participated in the non-gold plated NIRflex arm of the study, with 191 lesions treated and 19% of the patients had diabetes. The average diameter of the arteries was 2.74 mm, the average length of the stenoses was 10.3 mm and 65% of the stenoses were of a complex angiographic morphology (B2/C). The angiographic data gathered during the 6-month follow-up period showed a late-loss parameter averaging 0.65 mm. The clinical results at six months were as follows: no mortality TLR of 2.5% and a total MACE rate of 7.0% during the six month follow-up.
Figure 4 summarises the MACE and TLR results in those studies and in relation to the main drivers of restenosis outcomes in each study as well as in the context of our study findings.
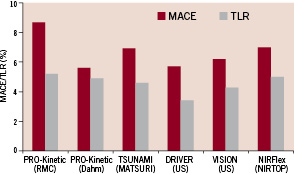
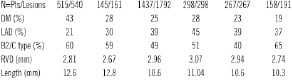
Figure 4. MACE and TRL rates in BMS reported studies.
It should be acknowledged that most patients included in these registries might have been of mild to moderate risk profile from a standpoint of procedural and restenosis risks. Nonetheless, these studies, as well as our registry data, demonstrate that, at least in a significant group of coronary patients, improved safety and intermediate-term efficacy results are certainly achievable using contemporary BMS platforms.
Study limitations
Our registry is limited by the following issues: 1) lack of a randomisation scheme to other BMS or DES counterparts, 2) retrospective mode of analysis, 3) single centre (albeit two hospitals) experience, 4) relatively short lesions and low prevalence of LAD lesions and/or multivessel and bifurcation interventions, and 5) lack of systematic angiographic follow-up. Moreover, as stated above, our results should be viewed in the context of the local clinical practice with ±40% of DES utilisation designed for patients at higher risk for restenosis. This ‘pattern of use’ would vary between countries and among centres and may have a major impact on the clinical results. Thus, our results are not necessarily applicable to all hospital environments and/or clinical scenarios.
Conclusions
The relatively low MACE and TVR rate that was observed in our registry indicates that the PRO-Kinetic stent can achieve excellent clinical performances in a large group of patients. Those encouraging results may provoke a discussion about the remaining role of contemporary BMS platforms and the frontiers of these devices in achieving optimal clinical results, even without the use of drug preparations.
Acknowledgements
This study was supported by an unrestricted educational grant of Biotronik Inc., Switzerland.

