Introduction
A novel magnetic resonance based technology was developed to address the drawbacks of conventional magnetic resonance imaging equipment in coronary applications. Intravascular MRI (IVMRI) is the first implementation of the technology and is designed for use in coronary catheterisation procedures. IVMRI exploits the unique magnetic field characteristics inherent in the self contained, miniature magnet and coil configuration, to provide contrast enhancement for measuring the molecular self-diffusion of water molecules in biological tissue1. The rate of the self-diffusion of water molecules in biological tissue provides accurate information as to the molecular environment that the water molecules experience. In the case of atherosclerotic plaque, which is comprised of two major groups namely fibrotic and lipid tissues, the self-diffusion of the water molecules provides a measure of the relative concentrations of the two groups present within the field of view of the IVMRI probe. The unique plaque classification method along with conventional MR capabilities and the miniature size of the probe have made this technology an ideal candidate for coronary wall imaging. The current generation catheter contains an IVMRI probe at the end of a custom delivery system (8F guiding catheter and 0.014” guide wire compatible) and is designed for use in standard catheterisation laboratories. Additional applications of the technology are currently being evaluated.
Theory of operation
The self-diffusion of water molecules in a measured volume is quantified in MRI by the apparent diffusion coefficient (ADC) value. In studies performed using conventional MR imaging equipment, it was shown that fibrous tissue (at 37ºC) yields an ADC of 1.45x10–9 m2s–1.2 as opposed to lipid-rich tissue which yields an ADC of ~0.26x10–9 m2s–1.2 The presence of calcium deposits within the measured volume is not detected by IVMRI due to the relatively low concentrations of hydrogen atoms available for MR excitation. The IVMRI probe contains an inherently strong static magnetic field gradient, in the order of 300 T/m, which provides a robust platform for Diffusion-Weighted Imaging (DWI) to measure ADC. In a spin-echo Carl-Purcell-Meiboom-Gill (CPMG) experiment and in the presence of such high-field gradients, diffusion becomes the dominant signal attenuation mechanism via loss of phase between the refocusing pulses3. ADC information is obtained from the rate of the spin echo signal decay in the CPMG pulse train characterized by the time constant (Tc) of the exponential decay. Assuming T2 (spin-spin relaxation time) >> Tc,4 Tc is inversely proportional to the ADC of the tissue. In the presence of the strong magnetic field gradients of the IVMRI probe and the large difference between the ADC values of fibrous and lipid tissues, significant differences in the Tc values between the tissues is acquired by the system. These differences are exploited to provide the tissue contrast of the IVMRI system by accumulating the signal under the exponential decay curve. Using this method a high accumulated signal corresponds to lipid-rich tissue and low accumulated signal corresponds to signals from fibrous tissue.
Since the distance of the measured volume from the arterial lumen has clinical implications, the position of the field of view (FOV) is significant5. The depth of the FOV in the IVMRI system may be set by changing the excitation frequency. The current generation has the depth of the FOV defined to penetrate between 50 and 200 µms (8 MHz) into the vessel wall thereby assessing lipid concentrations in the tissue volume close to the arterial lumen which may indicate fibrous cap thickness and subsequently the vulnerability of the plaque under interrogation.
The accumulated signal of a particular measurement is graphically represented in the IVMRI display. The display consists of colour mapped measurement sectors that indicate the lipid composition of the tissue within the field of view of the IVMRI probe. The colour mapping is derived from extensive human ex-vivo validation work. The quantified value provided by the system is the lipid fraction index (LFI) (Figures 1).
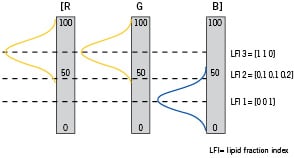
Figure 1. Graphical representation of the colour mapping technique. The three Gaussian distribution are obtained from measurements on fibrous (blue) and lipid-rich (yellow) aorta samples. Three examples are depicted by the dotted lines: a fibrous case (LFI 1), an intermediate case (LFI 2) and a lipid-rich case (LFI 3). In each case, the [R(red) G(green) B(blue)] color code is generated by summing the relative weight of the three distributions.
A sample screen display is shown in Figure 2.
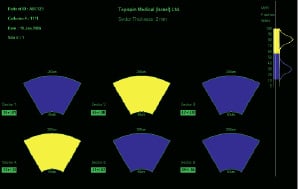
Figure 2. Sample IVMRI display with 6 measured sectors and lipid fraction index colour scale. The patient data is indicated in the upper left hand corner. Lipid fraction index scale distributions acquired in human ex-vivo experiments are indicated in the upper right hand corner.
System development and configuration
IVMRI has been in development since 1999 after initial feasibility analysis and experiments using conventional MR scanners. The size and scan time of the IVMRI probes have dramatically improved since the inception of the technology. From a 10 mm diameter alpha probe to a 1.5 mm diameter of the coming generation (to be released 2007). The IVMRI catheters are manufactured in a class 100,000 controlled environment room and have been approved for clinical trials in Europe and Israel.
Technical specifications
The system is comprised of three major components: a console, a catheter interface unit (CIU) and a catheter. The console is similar in size to IVUS consoles and provides the magnetic resonance pulse generation and amplification, echo acquisition and analysis, and user interface. The CIU is a small (26cmx15.5cmx6.5cm) electronic device that is placed on the patient table and connected to the console through a 4.5 meter cable. The CIU has a forward connector for interfacing the IVMRI catheter. System specifications are given in Table 1 and the mechanical configuration is illustrated in Figure 3.
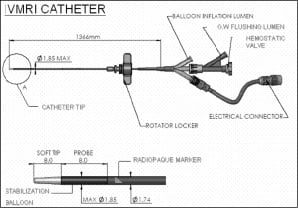
Figure 3. Full length of catheter and proximal ports (top) and enlargement of the distal end of the catheter (bottom).
Clinical application
The Topspin Medical IVMRI system is intended for magnetic resonance examination of vascular pathology. In particular, the system measures the lipid content of the intima or atherosclerotic plaque of the vascular wall.
The IVMRI system is designed for use in conventional catheterisation laboratories. The current generation is 8F guide catheter and 0.014” guide wire compatible. The system is introduced over a conventional 300 cm angioplasty guidewire into a coronary artery to a maximum distance of 5 cm from the orifice of the vessel. The system is designed for interrogation of non-obstructive lesions with an angiographically assessed minimum lumen diameter between 2-3.5 mm. The active tip of the IVMRI probe is positioned at the site intended for interrogation and the stabilisation balloon is inflated with a low pressure inflation device to a pressure of 1.4 atmospheres and the IVMRI recording is made over a period of 53 seconds. Following balloon deflation the catheter is rotated by a mechanical device producing a spiral withdrawal motion and the measurement is repeated at 5 additional, successively more proximal sites thereby providing comprehensive coverage of the plaque chosen for interrogation. The operator may access the IVMRI display at any point during the procedure.
Preclinical and clinical experience
The technology has undergone a series of preclinical studies in a range of models including ex-vivo human aortas, ex-vivo human coronaries6, in-vivo pigs and in-vivo atherosclerotic rabbits. The First-in-Man (FIM) clinical safety study has been completed and is reported in this issue of the journal. Thus far, a total of 61 patients have been enrolled into clinical protocols.
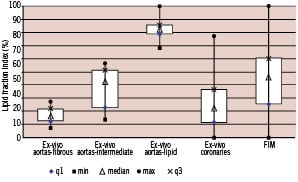
Figure 4. Distributions of IVMRI lipid fraction indices found in preclinical and clinical studies. Ex-vivo aortic plaques were characterised by histological analysis following assessment by IVMRI. The results show the contrast capabilities in the ex-vivo aorta studies as well as the wide distribution of findings in the ex-vivo coronaries and First-in-Man studies. q1= first quartile, q3= third quartile, max= maximum, min=minimum