Abstract
Background: To assess the safety and efficacy of percutaneous patent foramen ovale (PFO) closure for the prevention of recurrent stroke in high-risk patients.
Methods and results: Between January 2002 and March 2005, 40 patients (65% female), mean (SD) age 43 (10) years were identified using the following inclusion criteria: <60 years, recent brain infarction (<3 months), combination of PFO plus atrial septal aneurysm (ASA) and exclusion of any other thromboembolic cause. Percutaneous PFO closure was performed under general anaesthesia and under the guidance of transoesophageal echocardiography (TEE). Aspirin and clopidogrel were given for three months after PFO closure, followed by aspirin alone. Neurological examination was performed every three months.
Results: PFO closure was successful in all patients, using all devices: INTRASEPT™ in 34 cases, STARFlex™ in 5 cases and AMPLATZER™ in one case. At day one, transthoracic echocardiography (TTE) excluded malpositioning of the device or pericardial effusion. At 6 months, TTE and TEE confirmed satisfactory implantation of the device, with no spontaneous shunt. No device related thrombi was observed. Mean patient follow-up was 17±7 months (range 12-38) and was 100% complete. No patients suffered from recurrent stroke (neither transient ischaemic attack nor brain infarction).
Conclusion: This preliminary experience is encouraging for PFO closure with a 0% risk of stroke recurrence rate in a high-risk subset of patients (< 60 years, recent cryptogenic stroke, PFO plus ASA) and is safe using the current technology. Larger series with longer follow-up and randomised studies are still necessary in this setting before the completion of guidelines.
In young patients there is a strong causal relationship between patent foramen ovale (PFO) and the occurrence of a first cryptogenic stroke1. This relationship is even stronger when the PFO is associated with atrial septal aneurysm (ASA)2. However, the exact role of the PFO is not clear. Unless there is a clear identification of a thrombus formation inside the tunnel of the PFO, it can be viewed either as an innocent bystander or as the key determinant of the stroke. Regardless of these uncertainties, our therapeutic goal is to prevent recurrent neurologic events in such young patients. Despite aspirin therapy, the risk of recurrent stroke is rather low in patients with or without PFO alone (1% per year), but the risk is higher in those with the combination of PFO and ASA (4% per year)3. Therefore, two therapeutic options are offered, either long-term anticoagulant therapy (with an intrinsic haemorrhagic risk close to 1% per year) or a percutaneous closure and antiplatelet agents. The aim of this registry was to assess the safety of percutaneous closure and midterm clinical follow-up in this high-risk group of young patients (<60 years) who had had a recent cryptogenic brain infarction (<3 months) and a combination of PFO and ASA.
Methods
Patients
This prospective study was conducted between January 2002 and March 2005. All patients (<60 years) were referred by the neurological team, who had assessed the recent (<3 months) brain infarction (with positive cerebral computed tomography or magnetic resonance imaging) of unknown cause, magnetic resonance angiography excluding cervical artery dissection. Patients with any other identifiable cardiovascular thromboembolic risk were excluded, such as those with atherosclerotic plaques in the ascending aorta or extracranial arteries as determined by ultrasonography and magnetic resonance angiography; those in a prothrombotic state as determined by coagulation blood tests including protein C and S, antithrombin III, fibrinogen, antiphospholipid antibodies and APC resistance; and those suffering from paroxysmal atrial fibrillation as determined by continuous monitoring at the acute stage and subsequent 24-h Holter ECG monitoring. Patients with indications for oral anticoagulation were also excluded. The diagnosis of PFO plus ASA was ascertained using transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) and contrast study especially during TTE using a Valsalva manœuvre. TTE and TEE also excluded other intracardiac embolic causes as well as aortic debris. ASA was defined as an interatrial septum of abnormal mobility with protrusion of the septum into the left or right atrium of at least 10 mm beyond the baseline4.
Treatment procedure
The procedure was always performed under general anaesthesia and multi-plane TEE guidance. All patients were pretreated with aspirin (75 mg/day) and clopidogrel (75 mg/day). Thrombus formation during the procedure was prevented using an intravenous bolus of unfractionated heparin (100 UI/kg) followed by low molecular weight heparin (enoxaparin 1 mg/kg subcutaneously twice a day) for 48 hours. Subacute bacterial endocarditis prophylaxis was done using cefamandol (1.5 gr intravenously) at the start of the procedure and 2 hours later). Venous access was gained via the right femoral vein and the PFO was crossed with either a standard exchange guidewire alone or a 6F-multi-purpose catheter under fluoroscopic and TEE guidance. PFO closure was performed using a 12-F transseptal delivery sheath positioned in the left upper pulmonary vein. No balloon-sizing catheter was used. Different devices available during the study were implanted: INTRASEPT™ (CARDIA, Burnsville, Minn), STARFlex™ (NMT Medical, Boston, Mass), or AMPLATZER™ (AGA Medical Corp, Golden Valley, Minn). The type and the size of device were chosen according to atrial septal anatomy. The metallic-armed devices (INTRASEPT™ and STARFlex™) were mostly used due to the habits of the operators with no specific reasons. Residual shunt was evaluated using TEE with contrast study at the end of the procedure.
Follow-up
ECG, chest X-ray and TTE were performed systematically on day one. A combination of clopidogrel plus aspirin was pursued three months after PFO closure then followed by aspirin alone. Neurological examination was performed every three months by a neurologist in order to check for possible recurrent stroke. TTE and TEE were scheduled at six months. Residual (either spontaneous or provoked) right-to-left shunt was graded according to the amount of bubbles crossing the interatrial septum: grade 0 = none, grade 1 = minimal (1 to 5 bubbles), grade 2 = moderate (6 to 20 bubbles), and grade 3 = severe (> 20 bubbles)5. TTE was performed at one year in all patients with a residual shunt identified at 6 months.
Data analysis
Data were collected prospectively, and expressed as the mean (±SD). Analyses of the data included the description of peri-interventional adverse events as well as the recurrent neurological event rate during the follow-up period. No further statistical analyses were included.
Results
Patients
Forty consecutive patients met the inclusion criteria and underwent successful percutaneous PFO closure. Demographic characteristics are shown in Table 1.
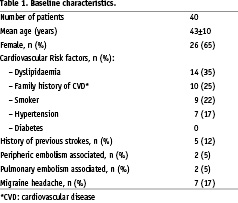
Among the patients, 5 (12.5%) had already had previous cerebral infarction as determined by cerebral imaging or clinical history, and 3 had concomitant pulmonary embolism. Migrainous headache with aura was present in 7 patients.
PFO closure procedure
All patients underwent successful percutaneous PFO closure, transseptal puncture was never used. Devices implanted were as follows: INTRASEPT(tm) in 34 cases, STARFlex™ in 5 cases and AMPLATZER™ in one case. Transient (< 5 min) ST-segment elevation in inferior leads was noticed during the procedure in 5 cases, with no conduction disturbances. In these patients, no CPK elevation occurred at 24-h. A spontaneous residual shunt was noticed in 7 cases. Neurological examination was normal in all patients immediately after interruption of general anaesthesia.
Follow-up
Mean patient follow-up was 17±7 months (range 12-38) and was 100% complete. The stroke recurrence rate was 0%: no patient had recurrent stroke, neither transient ischaemic attack nor brain infarction. On day 1, TTE confirmed the correct positioning of the device, with no pericardial effusion. No haemorrhagic complications occurred during in-hospital stay. At 6 months, TTE and TEE always confirmed the correct positioning of the device, with no spontaneous shunt. A residual right-to-left shunt was noticed in 9 patients (22%) treated with the INTRASEPT™ device graded as following: grade 1 (3), grade 2 (3), grade 3 (3). This residual shunt was detected using TTE and provoked Valsalva manoeuvre. At one year, the residual shunt was stable in 7 patients and disappeared in two (one with grade 2 and one with grade 3). No device-related thrombi formations was observed.
Discussion
These results suggest percutaneous PFO closure has a positive impact on stroke recurrence. On a midterm follow-up (17 months, and at least 12 months for each patient) in this high-risk patient group we observed no recurrent neurological events (neither stroke nor transient ischaemic attack). These results compare favourably with the natural history of these patients, since in the follow-up of one, well-monitored study, with a study population of 51 patients with both PFO and ASA while on aspirin therapy (300 mg/day), there was a 15.2% risk of recurrent stroke at four years, that is approximately 4% per year3. However, in this study the confidence interval was very large and most events occurred during the 4th year of follow-up. Longer follow-up duration is therefore needed before asserting the long-term clinical benefit of interventional endovascular strategies. Some strengths of our current study were: the careful work-up of stroke patients who were carefully selected as regards high-risk factors such as age, recent brain infarction, PFO plus ASA, thus ruling out any other detectable cause of embolic stroke; decision-making by the collaborative team including cardiologists and vascular neurologists, and follow-up carried out by neurologists to detect any recurrent neurological events. Thus, although small, this series shows in 40 patients that the one-year risk of recurrent neurological event (recurrence rate 0%) was encouraging, though not statistically different from the 4% in the 51 patients of the PFO/ASA study, due to the small number of these very carefully selected patients.
In our study, percutaneous PFO closure has been performed with a success rate of 100% with no major complication during hospital stay. A very low peri-procedural risk must be a prerequisite for a preventive therapy in a young population. The feasibility and safety of this procedure has currently been reported in numerous and large series and the reliability of the new device’s implantation must be now compared to medical treatment, especially long-term anticoagulation6-9. Transient ST-segment elevation in inferior leads observed during the procedure in 5 cases was probably due to air embolism in the right coronary artery through the transseptal sheath during device delivery. Even reported by all the authors, this phenomenon had no further consequences but a very careful and extensive flush of the sheaths must be done by the operators. The thrombogenic risk of the device itself exists but is very low, generally has no clinical consequences and is usually resolved under anticoagulant therapy10. Dual antiplatelet therapy with aspirin plus clopidogrel for a few weeks after device implantation is currently recommended to prevent thrombus formation. Careful verification with TTE and TEE showed no late thrombosis within six months after device placement in our study.
However, no prospective randomised trials comparing PFO closure and medical therapy have been reported so far, even in high-risk patients. With the new devices, invasive approaches are becoming easier, more reliable and safer, which explains the difficulties to end the ongoing randomised trials (RESPECT, CARDIA STAR, CLOSURE I trials), since most of the patients refused long-term anticoagulant therapy preferring PFO closure11. Recently, Windecker et al, compared PFO closure and medical treatment in a large but non-randomised study (150 had PFO closure and 158 were treated medically)12. At four years of follow-up, the rate of recurrent stroke was lower in the interventional group than those treated medically, although the difference was not statistically significant, 7.8% vs 22.2% (p=0.08). However the population of this study was inhomogeneous as it contained patients at low risk with PFO alone and patients older than 55 years. With respect to the additional presence of ASA, no difference was observed in recurrence rates between medically-treated and PFO closure groups.
Efficacy of percutaneous closure in the subset of patients with PFO and ASA has been recently reported in a study from two expert interventional cardiologic centres in Switzerland and Germany comprising 141 patients13. Long-term prevention of recurrent events appeared effective, since 95% of patients were free of recurrent transient ischaemic attack, stroke and peripheral embolism at four years. Results were similar (94%) in patients who were treated in the same way for PFO alone. The only predictor for recurrence was a residual right-to-left shunt after the intervention. At six month follow-up, residual right-to-left shunt was present in 14% (large in only 2%) and was unrelated to the size of the device implanted. This suggests that it is more important to achieve a complete closure of the PFO than to oversize device diameter in an attempt to entirely cover the ASA, which is probably not necessary.
The rate of residual shunt at 6 months in our study (22%) may be considered high, probably due to the use of forceful provocative manœuvres during TTE, which we considered more consistent than TEE in a sedated patient. One year TTE with contrast study must be repeated, and eventually we did not observed any increase in the shunt grade and a complete closure of the interatrial septal was observed in two, despite a severe shunt in one. Due to the small number of patients we cannot draw any conclusion regarding the type of the device.
Our study has several limitations: non randomised with a small number of patients and a relatively short follow-up. These results encourage us to focus on the indications of interventional procedures in this subset of patients, where ASA could act as a facilitator for embolism, by increasing the PFO diameter, by promoting the flow from the inferior vena cava, by local formation of the thrombus with subsequent embolisation, or even by increased atrial vulnerability14. The safety of the procedure and the fact that no recurrent neurological events have been observed supports the continuation of our current policies in high-risk patients while we await results from ongoing randomised trials.

