Abstract
Aims: False aneurysms can be treated surgically or by ultrasound guided manual compression. Another method is to inject thrombin into the aneurysm under ultrasound guidance. We evaluated safety and efficacy of this approach in a multicentre registry.
Methods and results: In 595 consecutive patients a pseudoaneurysm (593 femoral arteries, 2 brachial arteries) was diagnosed 0 to 250 days (median 3 days) after a catheter procedure. The diameter of the aneurysm ranged from 0.5x0.5x0.5 (LxWxD) to 8x11x16 cm (median 2x2x1.6 cm). 20 U to 4000 U (median 400 U) of thrombin solution were injected into the aneurysm under ultrasound guidance.
The procedure was technically successful in 587/595 (99%) patients.The aneurysms were thrombosed after the first injection in 531 (89%) patients. Thirty-eight (6%) patients needed a second injection and eight (1%) patients, a third injection because residual flow in the aneurysm was visible at follow-up. In four (0.7%) additional patients the thrombosis of the aneurysms was delayed and occurred only after 24 hours to seven days. Six (1%) patients surgery was performed after successful closure of the aneurysm to remove the resulting haematoma. The overall technical success rate was 99% and clinical success was achieved in 96%.
Eight (1%) other patients underwent surgery due to thrombin injection failure.
Complications occurred in nine patients (1,5%). Intravascular thrombus formation, deep venous thrombosis, pulmonary embolism due to deep venous thrombosis, transient paresthesia in the leg during injection.
Conclusions: Ultrasound guided thrombin injection is a safe, effective and rapid treatment of false aneurysms. Complications and recurrent pseudoaneurysms are rare.
Introduction
The incidence of iatrogenic femoral artery pseudoaneurysms is up to 0.2% following diagnostic procedures and up to 8% following interventional procedures, depending on the complexity of the procedure and periprocedural anticoagulation or anti-platelet therapy1-5.
Further risk factors for forming iatrogenic pseudoaneurysms are size of catheter (>7 Fr) or access sheath, older age of patients, multiple puncture of diseased (calcified) arteries, high or low puncture site, chronic haemodialysis, inadequate compression, hypertension and obesity34-36.
Complications of pseudoaneurysms include rupture, persistent pain and swelling around the affected site, neuropathy, distal embolisation, local skin ischaemia and necrosis, infection, and compression of adjacent vessels or nerves. The most serious complication, rupture, is related mainly to the size of the pseudoaneurysm sac36-38.
The classic treatment options are observation, surgical repair or ultrasound guided compression. Surgical repair is problematic, especially in patients requiring anticoagulation. It exposes the patient to the potential risks of anaesthesia, wound infection and other secondary complications, requires prolonged hospitalisation and results in higher costs. Despite this, there are still recognised indications for surgical repair: rapidly expanding pseudo-aneurysm; infected pseudoaneurysm; distal ischaemia caused by local pressure by the pseudoaneurysm on the femoral artery; neuropathy caused by local pressure on the femoral nerve; and failure of percutaneous treatment.
In the early 1990s, ultrasound guided compression was introduced as the first non-invasive treatment of pseudoaneurysms. However, this treatment has a high failure rate in patients who are anti-coagulated6 or in whom the pseudoaneurysm is larger than 4 cm in diameter7. Frequently it is not tolerated well due to pain. Also, it is very time consuming. Ultrasound guided compression is uncomfortable for the patient and challenging for the physician who is compressing the pseudoaneurysm. Contraindications to US-guided compression include: infection; coexisting large haematomas with impending compartment syndrome; limb ischaemia; skin ischaemia; excessive patient discomfort; and unsuitable anatomy.
Another non-invasive treatment of pseudoaneurysms is the ultrasound guided thrombin injection into the aneurysm. Thrombin originates from prothrombin and plays a central role in blood clotting. It converts fibrinogen to fibrin and activates prothrombin by means of positive feedback mechanisms and several coagulation factors (factors V and VIII)8. Thrombin injection leads to immediate thrombosis of the aneurysm. Of course the high thrombogenous potential of thrombin bears the risk of iatrogenic artery occlusion by artificial intravascular instillation.
This study evaluated safety and efficacy of this method in a multicentre registry.
Material and methods
Study design
This is a prospective multicentre registry. Enrolment commenced in January 1998. For the purpose of this publication the database was closed in December 31, 2005. Nineteen German hospitals participated. All data were collected according to the intention to treat principle.
Technique
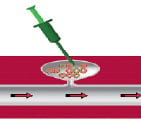
The procedure is performed under ultrasound guidance (5-7.5 MHz linear transducer). The size of the aneurysm in both the transverse and sagittal planes and roof of the pseudoaneurysm were measured and recorded. The skin overlying the pseudoaneurysm was prepared with a disinfectant. With the sonography transducer positioned over the pseudoaneurysm, a 20 G needle was inserted into the aneurysm with continuous ultrasound visualisation. Then a small amount of thrombin (50-100 U) was injected, followed by additional injections until a clot was forming and the blood flow in the pseudoaneurysm ceased. The neck of the aneurysm was not compressed during the thrombin injection. If residual flow was visible, additional thrombin was injected using the same technique (Figure 1a, 1b).

1. False aneurysm
2.Thrombin injection

3. After thrombininjection
Figure 1A. Technique of thrombin injection / Theoretical model.
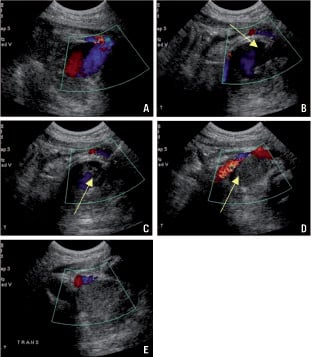
Figure 1B. a: Positioning of the ultrasound transducer over the pseudoaneurysm. b: Introduction of the needle. c: Thrombin injection. d: Thrombosis. e: Successful closure.
After documentation of complete closure of the aneurysm, the needle was withdrawn. Repeated colour and spectral ultrasound of the underlying femoral vessels were performed. The ipsilateral lower extremity pulses (posterior tibial and dorsalis pedis arteries) were evaluated before and after the procedure. A pressure bandage after thrombin injection was used only in case of residual flow in the aneurysm. Patients were instructed to remain in bed for 24 hours. Repeated duplex ultrasound was performed after 24 hours and after one week.
If flow was identified in the pseudoaneurysm at follow-up, the procedure was repeated. If the main body of the pseudoaneurysm was completely thrombosed, but the residual flow in the neck of the pseudoaneurysm was visible, thrombin was not injected into the neck because of the risk of distal embolisation.
Multiloculated pseudoaneurysms were treated by injection of thrombin into the deeper cavity (nearer to the puncture site). This resulted in spontaneous thrombosis in the superficial cavities without need for either compression or further injection.
All commercially available thrombin products were allowed and used depending upon availability (Table 1).

Study parameters at time of treatment included the initial catheterisation procedure, sheath size, number of days after catheterisation, complications, concomitant diseases, and recent history of anticoagulation or antiplatelet therapy were recorded.
Technical success was defined as thrombosis of the aneurysm after one or several thrombin injections, either immediately or within one week. Clinical success was defined as technical success without complications and without need for surgery.
Patients
From January 1998 until December 2005, 595 patients (m=335, w=260, mean age 70 years; age range 31-94 years), were included. The concomitant diseases inclusive of hypertension (n=301), hypercholesterolaemia (n=242), diabetes (n=93) and peripheral vascular disease (n=75) were registered. In 593 patients the femoral artery and in two patients the brachial artery was involved. If a false aneurysm was detected, thrombin injection was performed as soon as possible.
All patients in whom thrombin injection was attempted were prospectively and consecutively entered into the registry and followed according to the intention-to-treat principle.
The aneurysm was diagnosed 0-250 days (median 3 days) after the catheterisations.
Pseudoaneurysms were classified as simple (n=572) if they consisted of a single contained compartment directly communicating with the femoral artery or as complex if they consisted of multiple chambers (n=23) (Figure 2 a,b).
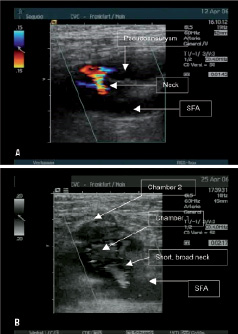
Figure 2. a: Simple aneurysm. b: Complex aneurysm.
The median length of the pseudoaneurysm (pseudoaneurysm dimension defined by flow) was 2.0 cm (range 0.5 to 8 cm), the median width 2.0 cm (range 0.5 to 11.0 cm) and the median depth 1.6 cm (range 0.5 to 16 cm).
Prior ultrasound guided compression was attempted and failed in 101 patients. Three hundred thirteen patients received aspirin alone, or aspirin and clopidogrel in combination. Sixty-three patients received aspirin / clopidogrel and heparin and 32 coumadin or heparin at the time of thrombin injection.
Results
We used 20 U to 4000 U of thrombin solution per treatment. The median dose of thrombin per treatment was 400 U.
Ultrasound guided thrombin injection successfully induced thrombosis in 587/595 (99%) pseudoaneurysms. The procedure was technically successful after the first injection in 531 patients (89%). In 46/587 (8%) patients, follow-up ultrasound on the day after the thrombin treatment revealed a recurrent pseudoaneurysm. In 38/46 patients, a second thrombin injection was performed. In 8/46 patients, a second recurrence occurred which was treated by a third injection.
In 4/587 patients (0.7%) thrombosis of the aneurysms was delayed and occurred only after 24 hours to 7 days.
In 6/587 (1%) patients, the aneurysm was closed but the haematoma was so large that it had to be removed surgically.
Eight other patients (1%) needed surgery because the aneurysm persisted after thrombin treatment. Technical success was achieved in 587/595 (99%) patients and clinical success in 572/595 (96%) patients.
Patients who received antiplatelet and anticoagulation in combination had significantly higher failure rate than other patients. The failure rate in the first group (patients who received antiplatelet therapy and anticoagulation in combination) was 6.3% (4/63) versus 1.3% (4/345) in the second group (patients who received antiplatelet therapy or heparin/coumadin only) (Fischer analysis p=0.0260).
We did not find a difference in therapy success between human and bovine thrombin. The success rate in bovine thrombin group was 98% and in human thrombin group 100% (Fisher analysis p=0.61). Human thrombin was preferred since it became available because of the potential risk of allergies.
Complications
Intravascular thrombus formation in the femoral artery occurred in three patients (0.5%). Two patients who had thrombo-embolic complications were asymptomatic, and their thromboses resolved immediately after intravenous heparin. One patient, who developed occlusion of femoral artery after thrombin treatment, had a non-occlusive stenosis in the right superficial femoral artery stenosis that trapped the embolised thrombin fragment and required surgical embolectomy.
Deep venous thrombosis occurred in three patients (0.5%). In one patient the thrombin was injected accidentally into the femoral vein resulting in a deep venous thrombosis. This was treated with fibrinolysis with rt-PA. Surgical repair of the femoral artery was necessary because of intense bleeding after fibrinolysis. The other two patients developed a deep venous thrombosis due to compression of the vein by the haematoma. They were treated with coumadin.
One patient (0.2%) developed a pulmonary embolism due to deep venous thrombosis.
Three (0.5%) patients complained about a transient discomfort in the leg during injection without further sequelae.
Discussion
Originally in 1986, Cope and Zeit13 and subsequently in 1987 Walker et al15, introduced the technique of percutaneous thrombin injection into the femoral artery. Kang et al14 reported a series of 21 consecutive cases with a 95 per cent success rate in 1997. Walker et al studied the thrombin ablation mechanism and found that thrombin was most effective in areas of stasis and described the thrombin injection via catheter15. Size of the pseudoaneurysm was not as important as the amount of flow present within the pseudoaneurysm.
The highly thrombogenous potential of thrombin bears the risk of iatrogenic artery occlusion by artificial intravascular instillation.
It can be assumed that the neck between the pseudoaneurysm and the artery after catheter intervention is small and is equivalent to the sheath size (a 5 Fr sheath has an outer diameter of 2 mm and an 8 Fr sheath 3 mm). These characteristics are favourable to prevent peripheral embolisation during thrombin injection16.
Optimal visualisation is the key to a successful procedure without complications. Thrombin injection should never be performed if visibility is poor and exact positioning of the needle is not possible.
Injection into the artery / vein should be strictly avoided. Sometimes in cases of complex pseudoaneurysms with diffuse haematomas and suboptimal visualisation, waiting for 24 hours to allow the reduction of soft tissue swelling may allow less impedance in imaging. Furthermore, we found that grey scale ultrasound is optimal for visualisation of thrombus formation during thrombin injection. We feel that colour / power Doppler at maximal sensitivity settings is optimal for evaluating residual flow within the pseudoaneurysm.
The use of a more dilute thrombin solution than previously reported with similar success rates is encouraging. Thrombosis remains fast, and obviously the higher concentration is not necessary for successful outcomes. Thrombosis occurred with an average dose of 400 units per patient, which is significantly less than in the previous report by Kang et al of 1000 units per patient with some patients receiving 2000 units14. We believe that this may actually reduce the potential for complications that involve the thrombosis of native arteries.
The complication rate with ultrasound guided thrombin injection is low (1.5%). In our study, we observed the following complications: thrombo-embolic complication, deep venous thrombosis, pulmonary embolism due to deep venous thrombosis and a transient discomfort in the leg during injection.
Intravascular thrombus formation in the femoral artery occurred in 0.5%. During the thrombin injection into the aneurysm, trespassing of thrombus formation from the aneurysm into the vascular lumen occurred. In one case, the thrombectomy of femoral artery was necessary due to occlusion of femoral artery.
Our 0.5% rate of thromboembolic complications is consistent with other reported rates19,27,28.
Venous thrombosis occurred in 0.5% in this series after thrombin injection. In one patient the thrombin was injected accidentally into the femoral vein. This was treated with fibrinolysis with rt-PA.
In another cases, the deep venous thrombosis resulted due to compression from large haematomas. One patient (0.1%) developed a pulmonary embolism due to deep venous thrombosis. All venous thromboses were treated with coumadin.
To minimise the risk of venous thromboses, we feel that surgical evacuation, when large haematomas are present, should be considered. Under these circumstances surgery is easier to perform than without thrombin injection because only the haematoma has to be removed without need to repair the artery. Similar complications were described by Hung et al29 in his study. On the basis of the results reported in the literature, the general conclusion of these authors invalidates the idea that thrombin injection for pseudoaneurysms should be avoided.
Successful brachial artery treatment is less well studied. In our series there were only two patients with pseudoaneurysms of the brachial artery. Both were treated successfully. However, there are reports in the literature about arterial thrombosis following thrombin treatment of aneurysm of small arteries, one in a 10-month-old child17 and one in an adult patient18.
Analysis of our failures discovered that anti-platelet therapy and heparin in combination during the procedure had a negative effect on treatment success and predispose to recurrent pseudoaneurysms. However even under these circumstances the success rate is high compared to ultrasound guided compression.
There remain questions about different outcomes between thrombin sources. In a study conducted by Vázquez et al30, the use of human thrombin was more efficient than bovine thrombin and the risk for allergy was potentially lower. In our registry we could not find a difference in efficacy between human and bovine thrombin.
Furthermore, an immunologic reaction to the foreign bovine thrombin is a well-understood risk of the procedure. Dorion et al found that 10% (12 of 120) of patients exposed to topical thrombin developed antibodies directed against bovine thrombin, and that patients with repeated exposure were eight times more likely to develop antibodies to coagulation factors43. Carrol et al documented the pattern of immunoglobulin (Ig) M and G antibody reaction to topical thrombin; peak levels of IgG and IgM occur at 68 weeks, with Ig G levels at 8 months 20-fold lower than the mean maximal level and Ig M levels returning to normal range44.
Krüger et al32 analysed the changes in systemic coagulation parameters implying that thrombin passes into the arterial blood stream and there is a risk of allergic complications. Patrick et al31 described a patient who experienced a systemic allergic reaction after percutaneous bovine thrombin injection.
We encountered no such complications in all 595 patients.
In recent years, several other studies demonstrated the high success rate of thrombin injection. In most studies the success rate ranges between 92% and 100%18-26 and corresponds with our results.
Our multicentre registry is the largest which has been published. It shows that thrombin injection is an effective and safe method for treating pseudoaneurysms after catheter intervention. The success rate was 99% in 595 patients. The rate of complications in our registry including the initial learning curve of participating centre is rare.
In conclusion, this study supports the postulate that treatment of iatrogenic pseudoaneurysms by thrombin injection is definitive, safe and efficacious.
Ultrasound guided percutaneous injection of thrombin can be regarded as the therapy of choice for the management of post-catheterisation pseudoaneurysms.

