Abbreviations
BA: bifurcation angle
DK-crush: double kissing crush
DMV: distal main vessel
EBC: European Bifurcation Club
FKI: final kissing inflation
MADS: (classification of the techniques) Main, Across, Distal, Side
PMV: proximal main vessel
POT: proximal optimisation technique
SB: side branch
SKS: simultaneous kissing stents
TAP: T and small protrusion
Introduction
Bifurcation disease accounts for up to 20% of all coronary disease treated by percutaneous coronary intervention (PCI).1 The best approach to manage a bifurcation to achieve optimal procedural outcomes and, more importantly, long-term success with low restenosis rates and low MACE rates is still heavily debated. This debate has predominantly stemmed from a lack of randomised data, which may explain why therapeutic strategies have been largely based on the personal clinical experiences of highly skilled operators practising in high-volume centres. Although the treatment strategy for bifurcation lesions has rapidly improved over the years, the lower procedural success, especially as the result of abrupt side branch (SB) closure with single stent strategies, together with the risk of thrombosis and restenosis associated with the complex two stent techniques, remains a predictor of adverse clinical outcome. The incidence of bifurcation lesions, the technical difficulties inherent in their treatment, the lower success rate, and higher complication and restenosis rates observed in this setting compared with non-bifurcation lesions have prompted intense research activity and have made coronary bifurcation stenting the focus of specific sessions in major interventional cardiology meetings.
The European Bifurcation Club (EBC) was founded in 2004 in order to devise a common terminology for the description and treatment of bifurcation lesions and to exchange ideas on the clinical, technical, and fundamental aspects of the specific treatment strategies implemented in this setting. EBC meetings are held annually. The fourth meeting was convened in Prague on September 26-27, 2008, with the agenda to reach a consensus on the current state of the art of percutaneous bifurcation treatment. The present report represents a synthesis of the findings from this meeting, and also incorporates a literature review from the field of bifurcation intervention. Topics covered in this consensus document include state-of-the-art in bifurcation stenting techniques, discussions of the anatomical changes at carina level during bifurcation stenting (carina shift) the relationship between bifurcation angle parameters and clinical outcome after intervention and the controversies in the technical approach to 0,1,0 left main lesions and 0,0,1 bifurcation lesions.
State-of-the-art in bifurcation stenting techniques
Bifurcations vary not only in anatomy (plaque burden, location of plaque, angle between branches, diameter of branches, bifurcation site) but also in the dynamic changes in anatomy during treatment (carina shift, dissection). As a result, no two bifurcations are identical, and no single strategy exists that can be applied to every bifurcation. Thus, the more important issue in bifurcation PCI is selecting the most appropriate strategy for an individual bifurcation and optimising the performance of this technique. The European Bifurcation Club has adopted in 2006 and promoted a simplified and universal classification of bifurcation lesions (the MEDINA classification)3. The Medina classification of bifurcation lesions was slightly modified in 2008 (Figure 1), following the fact that there is no disease at the level of the carina (see later).
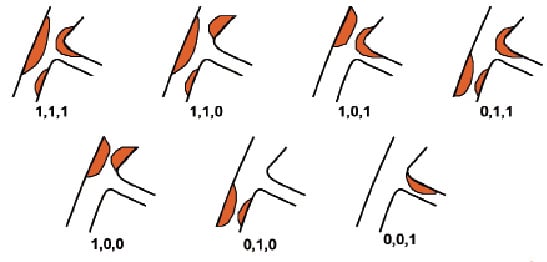
Figure 1. Modification of the Medina classification by removing plaques at the level of the carina. Courtesy of A. Medina.
In 2007, an accurate definition of each of the various techniques used – combined with a precise classification to facilitate their description – was proposed by Yves Louvard4. This classification of the techniques (MADS: Main, Across, Distal, Side) was based on the manner in which the first stent has been implanted (Figure 2), which often corresponds to a technical strategy related to the importance of the vessel treated first.
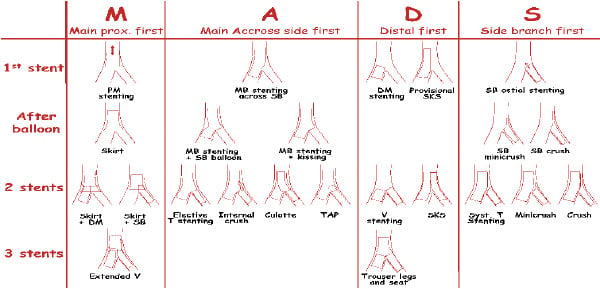
Figure 2. Summary of the MADS classification of techniques. Courtesy of Y. Louvard.
The present classification includes all potential technical strategies that have been published, reported or described during personal communications (EBC meeting) by describing four ways of beginning the procedure.4
The first family (M for Main)
This starts by stent implantation in the proximal main vessel (PMV) relatively close to the carina. This initial step may be followed by the opening of the stent towards both branches (SKIRT technique), with subsequent successive or simultaneous stent placement in one or both distal branches (extended Y technique).5
The Axxess stent (Devax Inc., Lake Forest, CA, USA) is a self-expanding dedicated bifurcation lesion drug-eluting stent, deployed at the level of the carina by means of a SKIRT technique and therefore belongs to the M family of techniques.6 The procedure for stent placement typically requires wiring of both branches of the lesion, followed by successful predilation of the PMV and the SB to provide space for the self-expanding stent. The stent is kept in place on the delivery system by a covering sheath, which is clearly visible under fluoroscopy. The stent is deployed by gently retracting the sheath once the stent is in place at the level of the carina. The stent has one radiopaque marker at the proximal edge and three markers at the distal edge to assist in accurate positioning and deployment at the target site. The Axxess stent’s conical shape and self-expanding property allow it to cover the irregular anatomy of a bifurcation at the level of the carina up to bifurcation angles of 70°. The stent is unique in that it spans both the PMV and the SB without obstructing access to either, and allows easy passage of additional stents in both branches. If needed, additional stents are implanted to complete lesion coverage. Accurate distal stent position is aided by the presence of the distal markers on the Axxess stent targeting a small amount (1 to 2 mm) of overlap. Post-dilation focus is on the distal branch stents, with attention to ensure full deployment at the overlap segments. Because the branch vessel stents are implanted distal to the carina, flow to the SB is unobstructed, and no strut deformation is induced by post-dilation.
Case selection (Table 1).
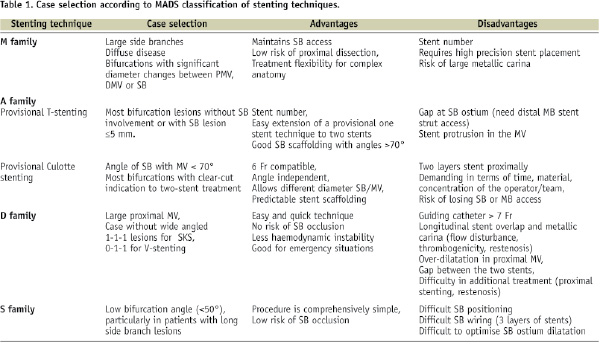
This technique is best suited for bifurcations with large side branches, with diffuse disease of the entire segment and bifurcations with significant diameter changes between PMV and distal main vessel (DMV) or SB. The advantages of this technique are that it maintains SB access and removes risk of SB occlusion, removes risk of proximal dissection and provides maximum treatment flexibility for complex anatomy. Disadvantages are the number of stents needed: the technique requires great precision in stent placement and a proximal stent could be positioned either too distal or too proximal to carina.
The second family (A for Across)
This starts with the stenting of the PMV to the DMV across the SB («provisional» side branch stenting strategy). This may be the first, and the last step of the procedure, but may also be followed by the opening of a stent cell with or without kissing balloon inflation towards the SB7, and, if necessary, by the delivery of a second stent in the SB in a T8, TAP (T And small Protrusion)9, culotte10 or internal crush configuration.11 This strategy is quick, safe, easy to perform, and has been shown to be associated with results that are comparable with a more complex approach in five randomised studies.
The provisional approach is performed as follows:
A 6 Fr guide catheter is generally used. After wiring both branches, the MV is predilated if required. There is a general consensus that SBs without severe calcification or long significant lesion (>5mm) do not require predilatation. Data from the TULIP and the SURF registry have shown that predilatation of the SB is not a predictive factor for “strut re-wiring” success and not a predictive factor for SB angiographic success. However, based on a prospective observational registry, Manuel Pan proposes a stepwise strategy of bifurcation stenting, with predilation of the SB also when the SB ostial stenosis is critical or if there is an unfavourable extreme angulation of the SB take off.12,13
Importantly, previous pathologic studies demonstrated that atherosclerosis occurs predominantly close to bifurcation, but carina (flow divider) involvement by atherosclerosis is extremely unusual. Those observations were also confirmed in vivo by IVUS pre-intervention evaluation of distal left main bifurcations (Oviedo et al. ACC 2008). Therefore, we should avoid the SB predilation and take advantage of the carina displacement/shift post-MV stenting since the re-crossing guidewire will cross the stent strut exactly at the carina level – carina cell (Figure 3).
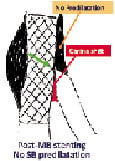
Figure 3. When SB is not predilated the guidewire will cross the stent strut at the carina level - carina cell. Courtesy of R. Albiero.
As opposed to this, SB predilatation can cause dissection and increases the risk of re-crossing a more proximal strut (with subsequent poor side branch scaffolding, as shown in Figure 4), through a dissection plane, and therefore increases the chance for potentially unnecessary SB stenting (Figure 5).
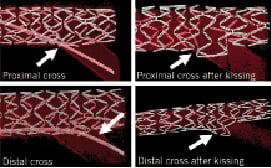
Figure 4. Proximal vs distal crossing in bench testing.
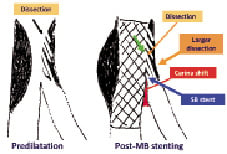
Figure 5. SB predilatation can cause dissection and increases the risk of re-crossing the more proximal strut through a dissection plane and, therefore, increases the chance for potentially unnecessary SB stenting.
The next step after MV predilatation is stent implantation in the MV across the SB, leaving the SB wire in place. If the angiographic results in MV and SB are satisfactory (there is no general consensus regarding the definition of satisfactory result at the SB ostium)14-16, the procedure is complete and the SB wire jailed behind the MV stent struts can be removed gently.13 If the result at SB ostium is not satisfactory, or if final kissing balloon is performed systematically, the SB is rewired with the MV wire (wire exchange) or a third wire is used for SB wiring before removing the jailed wire. In the provisional technique, wire crossing through the distal strut following MV stenting is strongly suggested because it creates better SB scaffolding than proximal crossing (Figure 4). In order to optimise SB access through the “carina strut”, the POT technique (proximal optimisation technique) has been proposed by Olivier Darremont during EBC 2007 (Figure 6).
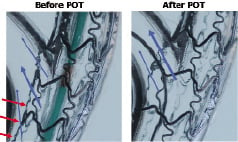
Figure 6. The proximal optimisation technique in bench testing. Courtesy of O. Darremont.
Optimisation of stent deployment proximal to the carina by using a short, half-size bigger balloon, or a spherical balloon (proposed by Remo Albiero), may help to access the most distal strut during wire exchange.
The jailed wire in the SB should always be left in place as a marker until complete re-crossing has been done. In addition, the jailed wire modifies favourably the angle between both branches and keeps the SB open. Several mechanisms have been proposed to explain SB compromise and ostial stenosis after stent placement in the MV, including: plaque shifting, ostial dissection, spasm at the SB ostium, stent strut coverage of the ostium, thrombus formation and carina displacement, as most probably the main mechanism. A recent analysis by Vassilev et al showed that the most powerful independent predictor of SB compromise is angle alpha, defined as angle between the axis of the parent vessel before the branch point and the SB axis at the point of divergence.17 Stent selection is also a very important parameter.
Stent selection for treating the MB of a bifurcation lesion is crucial for two reasons: stent diameter selection may play an important role during the procedure and the strut’s maximal circumference may influence the acute and long term result. The MB stent diameter should be selected according to Murray’s law (proximal MB diameter2=distal MB diameter2+SB diameter2). Therefore, if the stent diameter is selected according to the proximal MB reference, the carina shifting will be very important, with a high risk of SB occlusion or difficult SB wiring through the MB stent strut (Figure 7).
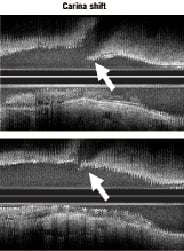
Figure 7. Carina shifting after MB stenting with an oversized stent (distal to the bifurcation). Courtesy of Koo et al.
This is why it is strongly suggested that we select the MB stent diameter according to the MB distal reference in order to decrease the risk of SB occlusion and then increase the stent size proximal to the carina (low risk of carina displacement) by using the POT technique and/or final kissing balloon inflation.
Mortier investigated the impact of different stent designs on the SB patency and access to the SB after stent implantation.18 Combining information of the ostium and stent cell size, he concluded that stent cell circumference should preferably be as large as the ostium circumference. Applying this criterium does not guarantee that all struts will be in contact with the tissue, but, it helps to select stents which have, at least, the potential to be sufficiently enlarged.
After re-crossing the SB, balloon dilation of the SB ostium and final kissing inflation (FKI) should be performed. FKI is proposed if the SB is dilated through the MV stent struts to correct MV stent distortion and expansion and provide better scaffolding of the SB ostium and facilitate future access to the SB.19,20 The CACTUS trial subanalysis showed that FKI was associated with a better angiographic results and lower MACE rates when complex stenting was performed and similar results were observed when using a more simple provisional SB stenting technique.21 However, before reaching general consensus on the need for FKI in a provisional strategy, we should wait for the results of the NORDIC-KISS trial which evaluates this problem in a randomised fashion.
If the result remains unsatisfactory after FKI (>75% residual stenosis, dissection, TIMI flow grade <3 in a SB ≥2.5 mm or FFR <0.75)14,22 SB stenting should be performed. According to randomised trials, a second stent in the SB may be required between 2% and 51% of cases.15,16,21,23,24 The ideal SB stenting rate is an unanswered question. FFR, or new techniques such as OCT, could be of value in the evaluation of SB result after balloon dilation. When the SB stenting is performed, the T technique is most frequently used6 (Table 1). It usually consists in positioning a stent at the ostium of the SB, being careful not to have the stent protruding in the MV. When the “carina strut” is open, optimal SB scaffolding is obtained with the MB stent (Figure 4) and there is no need for SB stent protrusion in the MB. Some operators leave a balloon in the MV to help precise positioning and sometimes inflate this balloon at a low pressure in order to help SB stent positioning. The TAP technique is a modification of the T-stenting technique and is based on an intentional minimal protrusion of the SB stent within the MV9. Final kissing inflation is performed using the balloon kept un-inflated into the MV before SB stenting.
In bifurcations with angles close to 90°C, T-stenting provides complete coverage of the SB ostium whatever the MB strut crossed. In Y shape angulation, crossing a distal strut is more crucial. Some operators prefer using the Culotte (or crush) techniques in order to be sure that SB scaffolding is obtained. The culotte technique leads to full coverage of the bifurcation at the expense of an excess of metal covering of the proximal end. The procedure starts with MV stenting, as in original description10, although the first stent can be deployed across the most angulated branch, usually the SB (inverted culotte).25 The non-stented branch is then rewired through the struts (the distal strut is better) of the stent and dilated. A second stent is advanced and expanded into the non-stented branch. Finally, kissing inflation is performed. An important technical consideration is that both branches should be well pre-dilated before stent deployment. After stenting, the operator should dilate each balloon separately at high pressure (>18 atm) and then inflate them together at 14-16 atm and deflate together.
Case selection (Table 1): The culotte technique can be proposed as a provisional SB stenting strategy in Y shape angulated bifurcation lesions, but also in most bifurcations with clear-cut indication to a two-stent treatment. Advantages of this technique are that it is 6 Fr compatible and very versatile: it is angle independent, allows different diameter SB/MV, and provides predictable stent cover. Disadvantages are that it is cumbersome, demanding in terms of time, material, concentration of the operator/team and it is sometimes annoying to lose access to one of the two vessels.
The third family (D for Distal)
This involves the distal branches and historically starts with simultaneous stent placement at the ostium of both distal branches (V stenting).26,27 A recent variant consists in creating a new carina by stent implantation in the proximal segments (Simultaneous Kissing Stent or SKS).28,29 A V stenting configuration can also be achieved by successive delivery of the stents; a “provisional” variant of SKS consists in delivering a single distal stent by inflating a balloon in the other branch.4 The procedure starts with adequate predilatation in order to allow stent expansion in the most symmetrical fashion. The size of the balloon and of the stents is chosen accordingly to the diameter of the vessels to be stented (1:1). Stent length is selected visually to cover the entire length of diseased segments (balloon for length). Two stents are positioned into the branches with a slight protrusion in the main proximal branch (V stenting). Different operators allow a variable amount of protrusion, creating sometimes a rather long (5 or more mm) double barrel in the PMV (SKS technique). Sometimes it is necessary to advance the first stent more distally into the vessel to facilitate the advancement of the second stent. After this step, the two stents are pulled simultaneously back to the bifurcation, making a V, and then into the proximal part of the main vessel to configure a Y, with the stem of the Y in the main vessel completely covering the proximal end of the lesion, one arm of the Y in the distal main vessel (covering the distal end of the main vessel lesion) and another arm in the side branch (covering the distal end of the side branch lesions). Once the position of the stents is confirmed and proximal stent markers are overlapped, the stents are deployed with simultaneous inflation and deflation at 16 atm or more for 10 to 20 seconds. In order to avoid stent slippage with simultaneous inflation, some operators propose that following accurate stent positioning, always verified in two projections, each stent is first inflated individually at 12 atm or more and then both stents simultaneously at 8-12 atm. The procedure is finished by FKI with short high-pressure balloons (12-16 atm).
Case selection (Table 1). The type of lesions considered most suitable for this technique are very proximal lesions such as bifurcation left main lesions with a left main artery which is short or free of disease. Ideally, the angle between the two branches should be less than 90°. It is also suitable for other bifurcations, provided the portion of the vessel proximal to the bifurcation is free of disease and there is no need to deploy a stent more proximally (0,1,1 lesions). It is quite intuitive how problematic the result of positioning a stent proximally to the double barrel may be with an inevitable bias towards one of the two branches and the high likelihood of leaving a gap. Advantages of this technique are that it is speedy, safe and non-complex procedure, with preserved access to both branches and less haemodynamic instability. Disadvantages are: the requirement of a large-size guiding catheter (at least 7 Fr), an artificial metallic carina which may lead to possible future troubles (flow disturbance, thrombogenicity, need for lifetime dual antiplatelet therapy, etc.), over-dilatation in the proximal MV, the possible risk of gap formation beneath the overlapped portion of the stent, difficulty in additional treatment, longitudinal stent overlapping in high-angle bifurcation and the distortion of the smaller stent promoted by a difference in size between the two stents.30,31
The fourth family (S for Side)
This involves strategies where the SB is stented first, either at ostium level32, or with relatively pronounced protrusion into the PMV33,34. The SB stent may be crushed with a balloon inflated in the MV, or a second stent may be deployed in the MV across the SB.
The S family comprises the largest number of techniques (systematic T stenting, crush stenting, mini-crush stenting, etc.). If systematic T-stenting is performed the following steps are recommended: after wiring the MV and the SB, predilatation is performed where needed. The next step is stenting of the SB with a balloon/stent in the MV. Following the removal of the side branch wire, the MV is stented. The SB is rewired, ostium of the SB is dilated and the procedure finalised with kissing balloon inflation.4,35 There are some disadvantages of stenting the SB first: accurate stent positioning at the ostium of the side branch may prove extremely difficult and if the SB stent protrudes in the lumen of the MV, passage of a stent into the MV may pose some technical difficulties.36 In that case, balloon inflation in the MV may create a space for stent insertion, but also, use of a buddy-wire or even rotablation have been proposed. To prevent this problem some operators have suggested that the stent should be deployed in the SB with simultaneous inflation of a balloon in the MV.8
The Crush technique has generated the largest number of variants.4 The classical Crush technique37 consists in the partial deployment of the SB stent in the PMV; the undeployed stent placed in the MV is subsequently deployed after removal of the SB wire. The main disadvantage of this technique is that it requires the use of a guiding-catheter of at least 7 Fr in diameter. The classical Crush technique is improved by final kissing balloon inflation38, which is now strongly recommended and some operators prefer to inflate a high-pressure balloon toward the SB before performing kissing balloon inflation21. The techniques known as Step Crush (Sheiban, personal communication) or Balloon Crush39 are identical and consist of crushing the SB stent with a balloon before advancing and deploying the MV stent. The techniques named modified balloon Crush40, double kissing crush41, sleeve technique42 are other variants of the Crush technique which may optimise stent deployment and apposition. When the SB stent is crushed on a short segment, the best denomination seems to be mini-crush as described by Galassi et al43 as compared to modified T stenting described by Kobayashi et al33.
A consensus of the European Bifurcation Club was achieved in that either mini-crush or DK-crush variants of the crush stenting should be used if this technique is selected for bifurcation treatment. In addition, as opposed to the provisional technique where a distal wire cross following MV stenting is proposed, in crush stenting “proximal” wire re-cross after crushing is suggested based on bench testing results because it creates better SB scaffolding than distal crossing.
As compared with the original study37 the mini-crush approach consisted of a minor retraction of the SB stent into the MV, so that the proximal marker of the side branch stent is positioned in the MV at a distance of 1-2 mm proximally to the carina of the bifurcation.43 Another difference consisted of crushing the SB stent with a balloon instead of the MV stent as in the standard approach; this is accomplished loading the balloon in the MV, covering the protruding SB stent segment and crushing it against the MV wall. The procedure is then completed, as in the standard crush, with FKI. The DK crush technique differs from the classical crush technique in that the part of the SB stent protruding into the MV is first crushed with a balloon in the MV, then kissing balloon inflation is performed, then the MV is stented and FKI is performed.41 The recent study by Ormiston et al44 gives us a better understanding about the downside of the crush technique if FKI is not performed and, more importantly, if it is not correctly performed.45,46 Additional insight into the results of the crush technique is provided by Costa et al in their intravascular ultrasound analysis.47 They observed that the area of the crush (i.e., the segment of stent overlap with three adjacent layers of stent) was most often the narrowest segment within the main vessel segment. Incomplete expansion of the crushed area was present in the majority of patients, even if FKI was performed, suggesting that at least high pressure dilation of the SB stent should be performed or better DK crush in order to optimise phasing between the struts of the two stents toward the SB.
Case selection (Table 1). Outcome after crush stenting is favourable in patients with a low bifurcation angle (<50°), particularly in patients with long side branch lesions, but also in most bifurcations with clear-cut indication to two-stent treatment.48 Advantages of this technique are that the procedure is comprehensively simple and the risk of SB occlusion is low. Limitations are that it is difficult to determine the position of SB stent, the SB wiring does not always lead to positive outcomes, dilation of SB ostium is limited due to the design of currently available stents as well as the fact of whether they are “in phase” or not.
Bifurcation angle
The take-off angle of the SB has been shown to have prognostic value in the procedural success and long-term outcomes of bifurcation lesion interventions and stenting in particular, and may have device specific implications.48,49 The methodology for angle assessment is variable and lack of consensus in published series make comparisons problematic.50 This parameter, perhaps more than others, is highly dependent on image acquisition requiring selection of the widest angle in the least foreshortened view. Three different angles could be measured for each bifurcation.4 It has been suggested (Y. Louvard, TCT 2003) that the angle between the PMV and the SB may be called Angle A. The degree of this angle has an influence on the accessibility of the side branch, which is frequently the reason for initially stenting the SB. Angle B is between the two distal branches (impact on the risk of SB occlusion during MB stenting). Angle C is between the PMV and the DMV. It has been shown to be related to the technical success rate of the Frontier dedicated stent. Dzavik et al defined bifurcation angle (BA) as the angle between the axis of the MV and the axis of the SB at its origin and, using the median BA of 50° as the cut point, divided patients into a low-angle group (BA <50°) and a high-angle group (BA ≥50°). A BA ≥50° was associated with a significantly higher rate of long-term MACE, and was independent predictor of MACE in patients undergoing crush stenting, along with the absence of a final kissing balloon inflation and poor baseline renal function.49 Dzavik also identified a potential interaction between the BA, successful post-crush kissing balloon inflation or at least a post-dilatation of the SB after crushing, and long-term outcome. The less favourable, long-term results after crush/culotte stenting in patients with high-angle bifurcations are likely secondary to the presence of adjacent areas of high shear stress, where platelet activation can occur, and low shear stress, where platelets can be deposited, mechanisms that are exaggerated under high-angle bifurcation conditions and that are likely further exacerbated by multiple layers of stent material that may not be optimally deployed.48
Three-dimensional (3D) reconstructions of coronary artery bifurcation lesions have the advantage over conventional two dimensional angiography to analyse geometrical changes that may occur following bifurcation treatment.51 Patrick Serruys presented during the EBC meeting in Prague preliminary results of 3D-QCA bifurcation angle analysis pre- and post- left main treatment (the Cardiop-B application by Paieon Inc, Rosh Ha’ayin, Israel). Advantages of 3D-QCA are objectivity, full automatic calibration which eliminates user induced error on calibration and avoidance of foreshortening and out-of-plane magnification. Reconstruction requires two clear, separate angiographic projections, being at least 30 degrees apart. Two bifurcation angles were calculated: proximal BA, defined as angle between proximal main vessel and SB, and distal BA, angle between distal main vessel and SB. Both are calculated in degrees separately for the end-diastolic and end-systolic frame and measurements were repeated pre- and post- procedure. By definition LCX is the SB. Using the SYNTAX left main PCI population (n=354 patients), the authors measured modification of left main bifurcation angle by systolic-diastolic motion and by treatment and evaluated the impact of the distal bifurcation angle on long-term outcome of the Thoraxcenter left main population 2000-2005 (n=157 patients). The principle findings of that analysis are: 1) there is a large variation in the left main bifurcation angulation parameters; 2) distal BA is affected by systolic-diastolic motion (during systolic motion there is an enlargement of the proximal angle and a reduction of the distal angle) and PCI treatment (after the PCI there is a reduction of the distal angle while the proximal angle is not modified significantly); 3) end-systolic values of distal BA affect the outcome (MACE) significantly and end-diastolic values exhibit a strong trend. This was the case both for the entire population, as well as for subgroups (DES, distal LM treatment).
The relationship between bifurcation angle changes after intervention (3D reconstruction using Cardiop-B application by Paieon Inc, Rosh Ha’ayin, Israel) and clinical outcome has been previously reported by Dvir et al.51 With the major limitation being the small number of patients treated with BMS, the authors concluded that major geometric changes after bifurcation stenting (especially where more than 20º change occurs) may be associated with increased risk of TVR during follow-up.
Further studies are warranted to confirm these findings as well as to determine if optimal mechanical and pharmacologic management can further improve the outcome of patients with coronary bifurcation lesions.
Controversies in the technical approach to 0,1,0 left main lesions and 0,0,1 bifurcation lesions
Approach to ostial LAD lesion
Left main 0,1,0 lesion (ostial LAD) is traditionally considered to be unfavourable for percutaneous intervention because of the technical difficulty and potential risk of serious complications. Two interventional strategies are traditionally used in this lesion subset: stenting the LM or precise stent implantation at the LAD ostium level. During EBC 2008, Alfonso Medina defended the approach of isolated stenting of the LAD ostium. Precise stent implantation indicates the implantation of the stent under angiographic/IVUS guidance without further manipulation of the stent. This procedure consists of scaffolding the counter-carina with a mild protrusion of the stent covering the ostium of the circumflex. The projection they propose is the spider view, and IVUS guidance was used in 70% of these cases. In the short axis, the characteristics of the LM and LAD were studied, revealing the remodelling index of 0.8. In the long axis view, the distribution of the plaque was analysed. Importantly, and in accordance with other recent investigations, no plaque was found in most patients at the level of the carina (inner part of the proximal LAD).
The technical description is as follows: the proximal marker of the balloon with the mounted stent should be positioned just proximal to the angiographic carina (the transducer of the IVUS catheter could be filmed when it is positioned at the carina level in order to have a reference for subsequent stent placement). Following LAD stenting, angiographic compromise of the ostium of the circumflex was observed in 19 patients (26%), but was significant (more than 50%) in only seven (10%). When IVUS images were compared before and after stent implantation, it seemed that angiographic compromise might be due to carina displacement. There was also one case where the stent jailed the circumflex ostium, but did not displace the carina enough to compromise it. IVUS evaluation of potential mechanisms of damage at the circumflex ostium revealed that in the IVUS long axis view examination showed a unique characteristic that was present at the level of the carina which the authors named the “eyebrow sign” (Figure 8).
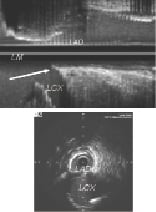
Figure 8. The “eyebrow sign” (arrow) at the level of the carina of the left main in the IVUS long axis view. Courtesy of A. Medina.
In 93% of the cases presenting with this sign, the circumflex ostium was damaged. On the other hand, in patients without this sign, ostial circumflex compromise was only observed in one out of 35 cases (3%). At follow-up, the target lesion revascularisation was 1% and the composite MACE 4%. Medina concluded that: 1) precise stent deployment for ostial LAD lesion is simple, safe and effective; 2) in most patients, the carina was free of plaque in the IVUS study; 3) floating stent struts are frequently observed, and do not relate to MACE, probably due to high flow at this level, and OCT at follow-up demonstrates endothelisation of the floating struts; 4) the presence of an “eyebrow” sign is a predictor of LCx ostial compromise, which does not need intervention in most cases; 5) carina displacement seems to be the main mechanism of this damage.
To the contrary, Carrie proposed left main stenting as the treatment approach to ostial LAD lesions. Disadvantages of precise ostial LAD stenting are the following: 1) if the device is positioned too proximally, it protrudes into the left main coronary artery that may compromise a LCx and make repeated intervention difficult; 2) if the ostial LAD lesion is not totally covered by the stent, acute recoil and late restenosis is expected. Therefore, optimal positioning of the stents is critical for the treatment of this lesion. Furthermore, with branch ostial disease there is frequent involvement of the distal LMCA, and thus the impending danger of incomplete lesion coverage if stenting is not extended to the involved left main. From this perspective, left main branch ostial lesion is necessarily a bifurcation disease and should be treated in a similar manner.
Therefore, ostial disease of LAD and LCx would ideally be treated percutaneously by stenting from the left main into the diseased main branch with provisional SB stenting. This approach provides complete lesion coverage. Final kissing balloon is suggested to manage carina shift and open stent struts, therefore maximising blood flow into the adjacent “jailed” vessel.
A consensus of the European Bifurcation Club was reached in that LMCA stenting into the diseased vessel is the proposed approach for most ostial LAD lesions. However, in cases with a large bifurcation angle and IVUS documentation of absence of disease in distal left main, a strategy with isolated ostial LAD stenting seems acceptable.
Approach to 0,0,1 bifurcation lesions: inverted provisional SB stenting or the “dogbone” technique?
Isolated SB ostial lesions (0,0,1), although not frequent, are very challenging lesions (especially in Y shape angulations). Different strategies are proposed to achieve the best treatment with “perfect” ostial coverage and no main vessel damage. Technical challenges are the problem of perfect ostial stent positioning, stent protrusion in the MV, or poor ostial coverage of the SB lesion and high risk of plaque or carina shift (in the MV).
To overcome these problems, Brunel has proposed the inverted provisional technique: cover the ostial lesion with a stent from the proximal main vessel to side branch and final kissing. The technique is as follows: two 0.014” wires in the MV and SB, with lesion predilatation, if necessary. The next step would be to implant a stent from proximal MV to SB. After wire exchange (cross the mesh from the proximal main vessel to the distal main vessel, taking a distal strut), the procedure is completed with a kissing balloon inflation. If necessary, a second stent could be implanted in the distal main vessel, as in a standard provisional strategy. Advantages of this approach are that ostial SB coverage is obtained in all cases and there is no stent misplacement related to cardiac movements, carina shift is corrected with systematic kissing and the procedure could be done with available “standard” DES. Disadvantages are related to differences in treated diameters, use of longer stent lengths and the presence of metal in the MV, as well as the possible compromise of the MV in case of strut crossing failure.
Another technique, proposed by Zehetgruber, is ostium stenting with the “dogbone” technique. Techniques to facilitate ostium stenting face the challenge of defining the point of stent deployment, while trying not to compromise the MV. The challenge of this manoeuvre is to define precise stent placement in the SB by keeping the inflated balloon in the MV (usually with balloon / vessel ratio 1:1), which, however, could create endothelial damage in the MV and does not assure precise SB stent position. Zehetgruber proposes the use of an undersized balloon in the MV (with the diameter calculated according to vessel diameter and bifurcation angulation), which would allow partial inflation of the delivery balloon from the SB stent inside the MV. This manoeuvre would help in better positioning the SB stent in order to achieve optimal ostial coverage. The advantage of this technique, in addition to a more predictable stent placement in the SB, is smaller endothelial damage inside the MV.
Alternative approaches are the use of a single short stent (precise ostial positioning is very difficult and complete ostial coverage is difficult/impossible, except for a 90 degrees bifurcation), type S treatment, shunt technique or dedicated stents.
Based on available data, no consensus was reached by the European Bifurcation Club regarding the best technical approach to 0,0,1 lesions and the decision was taken to set up a multicentre prospective consecutive EBC registry.
Conclusions
During the fourth European Bifurcation Club meeting consensus was reached on the following issues:
1. Atherosclerosis occurs predominantly close to the bifurcation, but carina (flow divider) involvement by atherosclerosis is extremely unusual.
2. SBs without severe calcification, or long significant lesions (>5 mm), do not require predilatation.
3. In the provisional technique, wire cross through the distal strut following MV stenting is proposed because it creates better SB scaffolding than a proximal crossing.
4. Carina displacement (not plaque shift) is the main mechanism of SB compromise following MV stenting, and stent diameter should be carefully selected according to Murray’s law.
5. The bifurcation angle between proximal MV and SB is an independent predictor of SB compromise.
6. Stent design and maximal stent cell size are important parameters in bifurcation stenting.
7. Mini-crush and/or DK-crush variants of crush stenting should be used for the S-family.
8. In crush stenting, proximal wire cross after crushing creates better SB scaffolding than distal crossing.
9. Final kissing balloon inflation is necessary in complex stenting, and may also improve angiographic and clinical outcomes in provisional stenting; we need further data from the ongoing randomised trial (Nordic-KISS).
10. The distal bifurcation angle is affected by systolic-diastolic motion and PCI treatment.
11. Angle modification after stenting may be associated with a worse clinical outcome.
12. End-systolic values of the distal bifurcation angle may affect the clinical outcome significantly.
13. A bifurcation angle ≥50° is associated with a significantly higher rate of long-term MACE after crush/culotte stenting.
14. In most cases, ostial disease of the LAD behaves as a left main lesion when approached interventionally, and the stenting technique can be selected and optimised by using IVUS.
15. In cases with large bifurcation angles and IVUS documentation of the absence of disease in distal left main, a strategy with isolated ostial LAD stenting seems acceptable.

