Abstract
Drug eluting stents (DES) have expanded the use of stents for the treatment of coronary atherosclerotic disease with significant reduction in restenosis rates. However, DES have been associated with late stent thrombosis (LST), especially when used for “off-label” indications. Although similar cellular processes control the early response after both bare metal (BMS) and DES placement, the more chronic response and time course of healing is markedly different between BMS and DES. There is persistence of fibrin beyond the 12-month period and re-endothelialisation is incomplete with some struts remaining uncovered beyond the 2-year period, a strong predictor for LST. While vessel wall injury correlated with restenosis in the BMS era, its impact has been minimised by the use of DES, which is likely related to the use of powerful antiproliferatives with prolonged release kinetics which profoundly inhibit the reparative response to arterial injury. However, at the same time, vessel injury secondary to drug toxicity or inflammation caused by polymer is observed following DES implantation. Non-erodable polymers induce granulomatous and hypersensitivity reactions in animal models and this has been observed exclusively with the use of Cypher stents in man. On the other hand the Taxus stent is associated with medial necrosis, positive remodelling and excessive fibrin deposition, all likely cytotoxic effects of paclitaxel. Both may lead to late stent thrombosis. Other factors that increase risk are penetration of the necrotic core, bifurcation stenting and malapposition.
Delayed vascular healing and late stent thrombosis
Once pivotal clinical trials comparing bare metal stents (BMS) to polymer based sirolimus (CypherTM) and paclitaxel (TaxusTM) drug-eluting stents (DES) demonstrated a significant reduction in-stent restenosis, DES quickly became the standard of care for the percutaneous treatment of symptomatic coronary artery disease. However, this overwhelming enthusiasm has recently been dampened by safety concerns regarding small but significantly increased rates of late (i.e. >30 days after implantation) DES thrombosis when compared to BMS especially in “off-label” use1,2 coupled with very high mortality of individuals suffering these events. Pathologic study of patients dying following DES implantation have revealed substantial impairment of arterial healing characterised by persistence of fibrin, minimal smooth muscle cell coverage of struts and incomplete endothelialisation when compared to BMS of similar duration3. We have also reported that a lack of endothelial strut coverage is the single best correlate of late stent thrombosis4. Several other pathologic risk factors were identified such as penetration of necrotic core, bifurcation, incomplete stent apposition, and hypersensitivity reaction.
The time course of arterial healing has been well characterised for BMS implantation5. At the early time point (<30 days), there is mild luminal thrombus with inflammatory cell infiltrates, consisting of polymorphonuclear leukocyte and macrophages. Platelets aggregation and fibrin deposition are observed for up to 30 days. T lymphocytes begin to infiltrate at two to four weeks and persist beyond the six months period. At the same time, smooth muscle cells begin to appear at 14 to 30 days accompanied by extracellular matrix consisting of proteoglycan and type III collagen. Completion of re-endothelialisation occurs by three to four months following BMS implantation. The neointima peaks at six to 12 months, and thereafter the neointimal volume decreases as type III collagen is replaced by type I collagen6. These observations are reinforced by clinical studies which showed that the amount of neointimal growth peaks at six to nine months and then decreases slowly up to three years following BMS implantation7.
There are certain similarities in the early cellular response between BMS and DES implantation as reported in human autopsy specimens8. Platelets and fibrin deposition is observed around stent struts along with focal acute inflammatory cell infiltrates following DES implantation in the first month. However, pathologic findings beyond 1-month duration are fundamentally different in DES compared to BMS. Smooth muscle cell proliferation is highly suppressed and is rarely seen until three to six months. This suppression of smooth muscle cells is more prominent in Cypher than Taxus and persists beyond one year. Fibrin deposition is more intense and diffuse in Taxus vs. Cypher and is greater as compared to BMS; it is commonly observed for both DES beyond 12 months. Poor endothelial cell coverage of the lumen is a consistent finding in some DES cases even as late as two to three years, which is in contrast to complete endothelialisation in BMS at three to four months. Uncovered struts, the strongest correlate of lack of endothelialisation, is frequently observed at later time point and is highly related to late stent thrombosis4. Recently, Awata et al in living patients reported poor stent strut coverage in sirolimus eluting stents compared with BMS using serial angioscopic analysis9, which is consistent with our histologic findings. Thus, delayed arterial healing is characterised by persistent fibrin deposition and incomplete endothelialisation, these changes in DES are observed for a long periods. These findings reinforce our pathologic observations that the time course of complete healing with DES in man remains unknown as a result so does the optimal duration of antiplatelet treatment.
Stent mediated vessel injury
Stent implantation is associated with vessel injury, which results in endothelial denudation, medial injury and/or fracture, plaque disruption and necrotic core penetration. The stent induced injury is very similar to that induced by balloon angioplasty alone except that the plaque is tacked back against the arterial wall by the expanding stent. It has been reported that medial injury and penetration of the necrotic core determine the extent of neointimal regrowth (i.e., restenosis) following BMS implantation10. Aggressive reduction of neointimal growth with the use of DES has been accomplished by the loading of cytotoxic and/or cytostatic drug on non-erodable polymers bound to stent surfaces. The drugs are released over a prolonged period of time (i.e. > 30-days for the Cypher stent), impacting vessel injury and neointimal formation with the latter highly minimised. However, the drugs and polymers themselves may also be toxic to the vessel wall. Initially these toxic effects were documented in animal models, where we reported medial necrosis accompanied by inflammatory infiltrate in paclitaxel and actinomycin D eluting stents11. These toxic effects were seen in high dose paclitaxel and all doses of actinomycin D and were often associated with positive vessel remodelling, arterial expansion with stent malapposition and stent thrombosis in the animal model. Similar findings were observed in the clinical study testing actinomycin D eluting stents with higher restenosis rate, and aneurysm formation12. Similarly, early preclinical paclitaxel eluting stent studies showed medial injury including medial necrosis and smooth muscle cell loss resulting in arterial dilation (i.e. positive remodelling)13,14. The degree of vascular injury is highly dependent on type of drug, drug dose and its release kinetics. Commercially available slow release Taxus stents in the porcine model demonstrate medial cell loss with fibrin deposition especially in overlapped regions15. In our autopsy cases with DES, fibrin deposition is more frequent in Taxus compared to Cypher stents. Because of underlying atherosclerotic plaque, the medial wall of the artery in man is usually not exposed to direct stent strut interaction. However, some human plaques with Taxus stent show severe fibrin deposition underneath malapposed stent struts, suggesting that drug induced cell death within the intimal plaque may occur (Figure 1A to C). Although it remains unclear whether fibrin deposition around stent strut is directly associated with stent thrombosis, it is likely to play an important role on the occurrence of late stent thrombosis and/or restenosis.
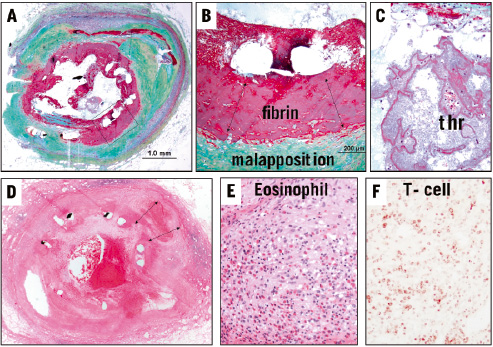
Figure 1. A to C are sections from a patient with Taxus stent implanted for 9 months. Note stent malapposition (B) and a luminal platelet rich thrombus (C) which was the cause of patients demise. In A, the boxed area which is shown in higher magnification in B are regions of stent malapposition with underlying fibrin rich thrombus separating the stent from the plaque. D to F are low and high power images from a patient with Cypher stent implanted for 18 months who presented with chest pain and died subsequently. Note stent malapposition (double arrows in D) and a diffuse inflammatory infiltrate involving the intima, media and adventitia. E is high power of the intima showing marked eosinophilic infiltrate along with lymphocytes. Special stains showed the lymphocytes to be T-cells (F, UCHL antibody).
Stent induced inflammation
On the other hand, severe inflammation is also one of the contributors of vessel wall injury. Hypersensitivity reaction is only observed in the Cypher stent, involves the entire stented vessel segment, which consists of eosinophils and T-lymphocytes infiltrates inducing internal and external elastic laminar break down, and positive remodelling. This phenomenon is not observed at early time points in man and animals while the drug is still eluting into the arterial wall because there is a potent anti-inflammatory effect. At the later time points beyond 90 days in the porcine animal model and at a median of 510 days (inter-quartile 312 to 740 days) in man, hypersensitivity reaction is observed in select patients treated with the Cypher stent8. The non-erodable polymer employed in this device is also known to promote inflammation when implanted in swine model. A case report from our laboratory initially described the occurrence of late stent thrombosis secondary to a hypersensitivity reaction in a patient at 18 months following Cypher stent implantation16. The morphologic changes were localised to the area of the stent and consisted predominantly of CD45-positive lymphocytes and eosinophils. In our series of human DES autopsy cases, we have observed five cases of Cypher stents with late stent thrombosis secondary to a hypersensitivity reaction and all cases showed positive vessel remodelling with extensive diffuse intimal, medial and adventitial inflammation (Figure 1D to F). Cypher non-erodable polymer consists of polyethylene-co-vinyl acetate (PEVA) and poly n-butyl methacrylate (PBMA), which have both been associated with hypersensitivity reactions17,18.
Both drug and/or polymer-induced vascular injuries in stented arteries are difficult to detect in the clinical setting because angiography only visualises luminal narrowing. However, many clinical studies utilising IVUS have shown positive remodelling following DES implantation19-21. An increase in external elastic membrane without a change in plaque area causes late acquired incomplete stent apposition. Early studies failed to recognise the impact of incomplete stent apposition on adverse clinical events16,22,23. However, Cook et al recently reported 13 cases of very late stent thrombosis (>1 year) with DES (eight Cypher and five Taxus) who had intravascular ultrasound examination at the time of angiography and compared these to non-thrombosed control patients. The thrombosed vessels had a high incidence of incomplete stent apposition (77% versus 12%, p=0.001) with significantly larger EEL area as compared to controls24. Ladich et al, in a small series of thrombectomy aspirates from patients with late stent thrombosis, have shown a higher percentage of eosinophils in thrombi aspirated from patients with Cypher stents versus those with BMS. The highest percentage of eosinophils was demonstrated in a patient with late stent thrombosis in a Cypher stent with positive remodelling of the vessel wall detected by IVUS and OCT25. It is likely that inflammation caused by DES plays an important role in vascular healing as well as late clinical outcome, therefore IVUS examination and/or other modalities and histologic examination probably give clues to the aetiology of late stent thrombosis.

