Abstract
Aims: Delays in initiation of treatment because of transportation of high-risk patients with ST-elevation myocardial infarction (STEMI) are associated with worse clinical outcome. Glycoprotein IIb/IIIa receptor inhibitors improve initial patency of the infarct-related vessel and reduce thrombotic complications in patients undergoing percutaneous coronary intervention (PCI).
Methods and results: The Ongoing Tirofiban In Myocardial infarction Evaluation (On-TIME) 2 trial is a randomised, double-blind, European clinical trial to evaluate the benefits of pre-hospital initiation of high-dose bolus of tirofiban, a glycoprotein IIb/IIIa receptor inhibitor, on background therapy of aspirin, unfractionated heparin and high dose clopidogrel, for patients with STEMI who undergo primary PCI. Eligible patients will be randomised 1:1 to pretreatment with a 25 µg/kg bolus and 0.15 µg/kg/min maintenance infusion of tirofiban or placebo. The primary endpoint is the extent of residual ST-segment deviation (defined as percentage of patients with >3 mm deviation of ST segment) 1 hour after PCI. The key secondary endpoint is the combined occurrence of death, recurrent myocardial infarction, urgent target vessel revascularisation, or thrombotic bailout at 30 days. The trial will continue until 958 patients are randomly assigned to treatment.
Conclusions: The On-TIME 2 trial evaluates whether pre-hospital initiation of high-dose bolus tirofiban is effective for patients with STEMI who are candidates to undergo PCI. This placebo-controlled trial will provide important evidence regarding the benefit of initiating a GP IIb/IIIa inhibitor, in combination with high-dose clopidogrel and unfractionated heparin.
Introduction
Early and complete restoration of blood flow in the infarct-related vessel (IRV) after acute myocardial infarction (MI) is associated with better survival and clinical outcomes.1-3 For those patients undergoing percutaneous coronary intervention (PCI), results of retrospective analyses suggest that outcomes are better if the IRV is open before the procedure (namely, thrombolysis in myocardial infarction [TIMI] flow grade 2 or 3).4,5
The benefits of PCI have been attributed to accelerated myocardial reperfusion within 90 minutes after the start of reperfusion therapy.6 Both short- and long- term outcomes are better after PCI than after fibrinolytic therapy for acute MI.6-9 Patients with an acute MI can safely be transported to a tertiary centre10; however, delays in initiation of treatment secondary to transportation of high-risk patients with MI may result in increased infarct size and reduced left ventricular function.11
Platelet activation and aggregation play a crucial role in the cascade of events leading to ST-segment elevation myocardial infarction (STEMI), as well as the thrombotic complications that can accompany PCI. Insufficient inhibition of platelet aggregation at the time of PCI correlates with increased likelihood of major cardiovascular adverse events after PCI.12 Antiplatelet therapy, therefore, is an important component of medical therapy for patients with STEMI.
Glycoprotein (GP) IIb/IIIa receptor inhibitors, which block the final common pathway leading to platelet aggregation, improve initial patency and reduce thrombotic complications in patients undergoing PCI, including both balloon angioplasty and stent implantation.13-16 The efficacy of GP IIb/IIIa inhibitors for high-risk patients with acute coronary syndrome is well established.17,18 Triple antiplatelet treatment, including aspirin, a thienopyridine, and a GP IIb/IIIa inhibitor, is recommended for high-risk patients with non-ST segment elevation acute coronary syndromes; however, the optimal timing of GP IIb/IIIa inhibitor administration has not been established.17,18 Similarly, the optimal timing of GP IIb/IIIa inhibitor administration has not been established for patients with STEMI who are candidates for PCI,19 and identifying the most effective antiplatelet agent(s), dosage, and timing of administration in this setting remains the subject of intense clinical investigation.
Preliminary findings suggest that early administration of a GP IIb/IIIa inhibitor is associated with improved outcomes after PCI.20-24 A meta-analysis of all randomised studies of early versus late administration of a GP IIb/IIIa inhibitor showed that patients with STEMI who received abciximab or tirofiban early (before catheterisation) more often had a patent IRV at initial angiography as compared with patients who received the GP IIb/IIIa inhibitor late, just before PCI (Figure 1).20-25
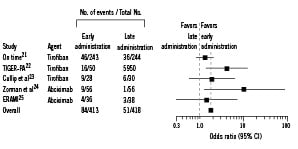
Figure 1. Odds ratios for thrombolysis in myocardial infarction (TIMI) grade 3 flow with early vs late administration of glycoprotein IIb/IIIa inhibitors. Overall odds ratio, 1.85 (95% confidence interval [CI], 1.26-2.71; P<0.001). Breslow-Day test for heterogeneity, P=0.12. Reprinted with permission from Montalescot et al, 2004.20 On-TIME indicates ongoing tirofiban in myocardial infarction evaluation trial21; TIGER-PA, tirofiban given in the emergency room before primary angioplasty trial22; ERAMI, early reopro administration in myocardial infarction trial.25
The On-TIME pilot trial is the largest randomised trial showing that pre-transportation initiation of the GP IIb/IIIa inhibitor tirofiban is safe and is associated with improved initial patency of the IRV (TIMI flow grade 2 or 3).21 However, results of recent studies indicate that the bolus dose of tirofiban used in the On-TIME trial is too low for optimal inhibition of platelet aggregation.26-30 In the TRIPAS (Tirofiban or Reopro In Primary Angioplasty) study, adequate levels of platelet inhibition were obtained only with a higher bolus dose of tirofiban.29 The potential benefit of a higher bolus dose of tirofiban, given very early after symptom onset, often in a pre-hospital setting, for patients with STEMI is not described.
Study objectives
This prospective, randomised, double-blind, placebo-controlled, multicentre, European trial will evaluate the effect of pre-hospital initiation with a high bolus dose of tirofiban, administered in addition to aspirin, unfractionated heparin, and clopidogrel 600 mg, on the extent of residual ST-segment deviation (defined as percentage of patients with >3 mm deviation of ST segment) at 1 hour after PCI in patients with STEMI.31 Bailout tirofiban is to be used as thrombotic rescue. Secondary study objectives are to evaluate the effects of this treatment regimen, as compared with placebo and in addition to aspirin, heparin, and clopidogrel 600 mg, on the incidence of death, recurrent MI, urgent target vessel revascularisation (TVR), or thrombotic bailout combined at 30 days; of major bleeding using the most recent TIMI criteria32; of TIMI 3 flow of the IRV at initial angiography; and of normal myocardial perfusion as assessed by myocardial blush grade scoring33 immediately after primary angioplasty.
Study design and patients
Recruitment has commenced since June 2006 at high-volume PCI centres in Germany, and the Netherlands. These centres, all of which perform primary PCI as the default reperfusion method – available 24 hours/day and 7 days/week, with surgical back-up – will enrol consecutive eligible patients. Emergency transportation will be provided as necessary to ensure arrival of the patient to the catheterisation laboratory within 2 hours of randomisation. Recruitment and randomisation in ambulances will begin only after at least a 6-month period of training in pre-hospital infarct diagnosis and care.
Patients
The study population will consist of 958 eligible patients with STEMI who are candidates to undergo primary PCI. Eligible patients are men and women 21-85 years of age with symptoms of acute MI of >30 minutes but <24 hours and ST-segment elevation of >1 mV in 2 adjacent electrocardiogram (ECG) leads, with cumulative ST-segment deviation of ≥6 mm. In addition, to be eligible, patients must be appropriate candidates for PCI within 2 hours after randomisation.
Patients who have left bundle branch block on ECG, who are unable to give informed consent, or who have life expectancy of <1 year are excluded from the study, as are those in cardiogenic shock (systolic blood pressure ≤80 mmHg for >30 minutes) or needing an intra-aortic balloon pump. Other exclusion criteria include treatment with thrombolytic therapy within 24 hours, warfarin within 7 days, another GP IIb/IIIa inhibitor within 30 days, or another investigational drug or device within 4 weeks; known severe renal dysfunction (glomerular filtration rate <30 ml/min or serum creatinine >200 mmol/L [>2.5 mg/dl]); confirmed or persistent severe hypertension (systolic pressure >180 mmHg and/or diastolic pressure >110 mmHg); a contraindication to anticoagulation or increased risk of bleeding; and low haemoglobin (<11 g/dl) or haematocrit (<33%). Pregnant women and those who are breast-feeding are not eligible for the study.
Informed written consent will be obtained before initiating any study procedure. If the clinical situation prevents a written consent, then a verbal consent will be obtained in the presence of a non-relative witness and a written consent will be obtained immediately after the PCI procedure or as soon as the patient is capable of giving consent. The study protocol has been approved by all local ethics committees.
Randomisation and study treatment
Patients will be randomly assigned to pre-hospital treatment with tirofiban (25 µg/kg bolus and 0.15 µg/kg/min maintenance infusion for 18 hours post PCI) or placebo by receiving a consecutive randomisation number, as assigned to each investigator. Patients will be stratified by intended place of recruitment (ambulance, referral centre). The stratified randomisation will be generated within each investigative site using random permuted blocks, with a 1:1 allocation of treatments. The referring physician, ambulance personnel, and/or the investigator will complete the enrolment procedures. The study flow chart is shown in Figure 2.
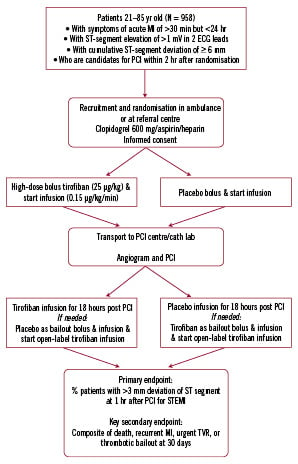
Figure 2. On-TIME 2 flow chart.
Concomitant medication
In the ambulance, all patients will also receive a bolus of unfractionated heparin (5000 IU) intravenously together with aspirin 500 mg intravenously and clopidogrel 600 mg orally. Following this a bolus of tirofiban or placebo will be injected followed by an infusion of tirofiban or placebo. Before PCI, the activated clotting time will be assessed once; if it is <250 seconds, then an additional bolus of 2500 IU of unfractionated heparin will be administered.
Clopidogrel 75 mg daily will be continued for at least one month after placement of a bare metal stent and at least 12 months after placement of a drug eluting stent. Oral, sublingual, topical, or intravenous nitrates may be administered at the discretion of the treating physician. Additional medical therapy with β-blockers, nitrates, calcium antagonists, and cholesterol-lowering agents is also recommended, in accordance with current practice guidelines.34,35
Warfarin, dipyridamole, or nonsteroidal anti-inflammatory agents should not be instituted until completion of the study drug infusion. No other antiplatelet (except aspirin and clopidogrel) or anticoagulant drugs may be administered until completion of the study drug infusion. The use of low molecular weight heparin is discouraged except as indicated by circumstances, for example, prophylaxis of venous thrombosis. Non-study medications received up to 10 hours after completion of study drug, any hormonal replacement agents, and the type of dye used for the angiographic imaging will be recorded on the appropriate case record forms.
Study procedures
Blood pressure and heart rate will be monitored before transportation, 15 minutes before PCI, 24 hours after PCI, and at other times according to each institution’s standard procedures. A medical history will be recorded and a complete physical examination performed before angiography. A standard 12-lead ECG will be recorded at the time of diagnosis, before angiography, and 30 and 60 minutes after PCI, as outlined in Appendix A. Standard haematology and biochemistry tests, as well as cardiac troponin T levels will be determined before angiography and at regular intervals after PCI.
Coronary angiography and primary coronary angioplasty (PCA) will be performed according to each institution’s guidelines and standards.
Criteria for bailout use of tirofiban (thrombotic bailout)
During or after PCI the operator may decide to administer open-label tirofiban for the following indications: Decrement in TIMI flow grade (TIMI flow grades of 0-2 or slow reflow), dissection with decreased flow, distal embolisation, side branch closure, abrupt closure of the culprit vessel, clinical instability, and prolonged ischemia. Bailout tirofiban is administered as the high-dose bolus, namely 25 µg/kg bolus and 0.15 µg/kg/min maintenance infusion for 18 hours. To maintain the blinding of the initial treatment assignment and the content of the primary infusion, bailout vials will be included in the medication box and are blinded. When a bailout vial is given, the study infusion has to be replaced by open-label tirofiban (not supplied).
Follow-up
Patients will be seen in the outpatient clinic at 30 days and contacted by telephone at one year after PCI and as frequently as considered necessary by the investigator or referring physician during the 1-year period.
Hypotheses
We hypothesise that pre-hospital initiation of high-dose bolus of tirofiban, on background therapy of aspirin, heparin, and clopidogrel, will result in a lower extent of residual ST-segment deviation (defined as percentage of patients with >3 mm deviation of ST segment) at one hour after PCI for STEMI, as compared with placebo. Secondary hypotheses are that, compared with no pretreatment with tirofiban in addition to aspirin, heparin, and clopidogrel, this pre-hospital treatment with tirofiban 1) will result in a lower combined incidence of death, recurrent MI, urgent TVR, or thrombotic bailout at 30 days; 2) will not result in a higher incidence of major bleeding using most recent TIMI criteria32; 3) will result in a higher incidence of TIMI 3 flow of the IRV at initial angiography; and 4) will result in a higher incidence of normal myocardial perfusion as assessed by myocardial blush grade scoring immediately after primary angioplasty.
Endpoints
The primary efficacy endpoint is the extent of residual ST-segment deviation (defined as percentage of patients with >3 mm deviation of ST segment) at 1 hour after PCI.31 The key secondary endpoint is the composite of death, recurrent MI, urgent TVR, or thrombotic bailout, at 30 days follow-up. Other efficacy endpoints include the incidence of TIMI 3 flow of the infarct related vessel (IRV) at initial angiography36 and the incidence of normal myocardial perfusion as assessed by myocardial blush grade scoring immediately after primary angioplasty.33
A blinded independent clinical endpoint committee (CEC) will adjudicate all clinical endpoints except death. Death will be defined as all-cause mortality. MI within 30 days after completion of PCI is defined as a new increase of creatine kinase MB (CK-MB) ≥3 times the upper limit of normal, present in two separate blood samples, and whether or not accompanied by chest pain and/or ECG changes. Early recurrent infarction is defined as a decrease in CK-MB of at least 50% of the upper limit of normal from a prior peak level to a valley followed by a new increase with a value above the sum of the preceding valley and 3 times the upper limit of normal. Urgent TVR during the hospitalisation period will be defined as a new episode of ischaemic signs or symptoms at rest with documentation of a new ST-segment shift ≥0.05 mV (0.5 mm) on a 12-lead ECG that necessitates an unplanned coronary intervention or coronary artery bypass grafting. “Recurrent ischaemia leading to revascularisation” after the hospitalisation period will be defined as readmission to hospital, after discharge from the acute hospital, within 30 days of randomisation for an episode of ischaemic signs or symptoms at rest that requires cardiac catheterisation and revascularisation before discharge. The use of open-label (high-dose bolus) tirofiban during or after PCI for thrombotic bailout is based on predefined indications as described above in text of paper. Stroke will be adjudicated by this blinded clinical events classification committee and will be defined as an acute new neurological deficit ending in death or lasting >24 hours not caused by another readily identifiable cause such as trauma.
The safety endpoints of interest include the rates of haemorrhage, transfusions, stroke, thrombocytopenia, and serious adverse events. An independent Data Safety Monitoring Committee (DSMC) is responsible for identifying safety issues and making any recommendation regarding modification or termination of the study to the Executive Committee. Bleeding will be assessed by use of the TIMI criteria.32,37 Major bleeding is defined as follows: clinically significant overt signs of haemorrhage associated with a drop in haemoglobin of >5 g/dL (or, when haemoglobin assessment is not available, a decrease in haematocrit of >15%). For patients undergoing coronary artery bypass graft (CABG) surgery, the rate of surgical re-exploration for bleeding and the postoperative volume of blood loss will also be evaluated.
Sample size and statistical hypothesis
Residual ST-segment deviation of >3 mm is assumed to be present at one hour after PCI in 50% of unselected infarct patients who have not received GP IIb/IIIa inhibitor treatment in addition to aspirin, heparin, and clopidogrel38. Treatment with the high-dose bolus of tirofiban is assumed to decrease by 20% the incidence of residual ST deviation of >3 mm, from 50% to 40%. On the basis of these assumptions, with 80% power and an alpha of 0.05, 814 (2x407) patients are needed to show superiority of tirofiban treatment. To account for incomplete or uninterpretable ECG data and false positive ECG diagnoses for approximately 15% of patients, 958 patients will be randomised. With this sample size, the study has 68% power to detect a 40% relative reduction (from 13% to 8%) in the combined incidence of death, reinfarction, urgent TVR, or thrombotic bailout.
Data analysis
All analyses will be performed using a modified intention-to-treat approach and will include all patients who are randomised and receive any amount of study drug, but for whom a false positive infarct diagnosis is excluded. The primary efficacy variable, the percentage of patients with >3 mm cumulative ST deviation at 1 hour after PCI, will be analysed using the χ2 test or Fischer exact test. In addition the χ2 test for trend will be used to analyse the percentages of patients in each of the four prespecified groups of residual ST-segment deviation as follows: 0 mm=normalised ST segment – no residual ST-segment deviation; 1-3 mm=residual ST-segment deviation between 1 and 3 mm; 4-6 mm=residual ST-segment deviation between 4 and 6 mm; >7 mm=residual ST-segment deviation more than 7 mm. All ECGs will be analysed by an independent core lab that will be blinded to the randomisation. The sum of ST-segment deviation in all 12 leads will be measured 20 ms after the end of the QRS complex with a calliper.
Data management
The data will be managed by Diagram BV (Diagnostic Research and Management), Zwolle, The Netherlands, blinded to treatment assignment. Data will be collected using case report forms, Clinical Endpoint Committee (CEC) adjudication forms, and the Diagram site descriptor database. The only individuals with access to unblinded event rates before database lock will be the DSMC and a different/independent statistician involved in preparation of DSMC reports.
Study organisation
Members of study committees are listed in Appendix.
Executive Committee: The Executive Committee, which represents members of the academic group and sponsors, will be responsible for the overall design, conduct, and supervision of the trial. It will adjudicate policy issues among the various constituencies of the trial and will be responsible for reviewing the progress of the trial at regular intervals to ensure patient safety and trial integrity. It will also review protocol amendments.
Steering Committee: The Steering Committee will be responsible for providing clinical guidance on protocol logistics, study implementation and conduct.
Data Safety Monitoring Committee: An independent DSMC, composed of experienced cardiologists and a statistician, will be responsible for reviewing the progress of the study at regular intervals to ensure patient safety and study integrity. The DSMC will meet when 30% and 75% of patients have completed the 30-day study unless it is deemed that additional monitoring is necessary. The chairman of the DSMC will monitor all adverse events and serious adverse events on a continual basis and may request an unplanned review of all safety data by the entire DSMC, with full unblinding, if a safety concern arises.
Clinical Event Committee: The independent CEC will systematically identify and adjudicate suspected endpoint events according to prespecified criteria, described above. In addition, while reviewing records of a patient who was triggered for an event, the CEC may occasionally discover another potential event to adjudicate. Events that will be adjudicated include recurrent MI, urgent TVR, thrombotic bailout, and bleeding. Members of the CEC will remain blinded to treatment throughout the adjudication process and the study. The CEC-adjudicated data will be used in the final efficacy and safety analyses unless otherwise stated.
Publication Committee: The Publication Committee will be composed of representatives of the participating academic and other institutions and will be chaired by the Executive Steering Committee. All proposed analyses, presentation, and publications will be submitted in advance to the Publication Committee and the sponsor for comment.
Discussion
The On-TIME 2 trial is designed to evaluate the benefits of an early up-front, high-dose bolus of tirofiban, as compared with placebo, in addition to aspirin and a high loading dose of clopidogrel (600 mg), in patients with STEMI who undergo primary PCI.
Choice of tirofiban dosage
The bolus dose of tirofiban (10 µg/kg) given in TARGET (Do Tirofiban and ReoPro Give Similar Efficacy Outcome Trial)39 is now considered to have been insufficient for optimal inhibition of platelet aggregation.26-28 After initiation of tirofiban as a 10 µg/kg bolus followed by a maintenance infusion of 0.15 µg/kg/min, the inhibition of maximal platelet aggregation induced by 20 µM adenosine diphosphate ranged from 61% to 66% at 15 to 45 minutes as compared with a range from 90% to 94% at 15 to 45 minutes after initiation of abciximab (0.25 mg/kg bolus followed by 0.125 µg/kg/min infusion).26 However, a single bolus dose of tirofiban of 25 µg/kg followed by a maintenance infusion of 0.15 µg/kg/min results in consistent and effective inhibition of platelet function to a degree similar to that produced by the abciximab regimen in TARGET.27,28
More recently, results of clinical trials indicate that the high-dose bolus of tirofiban improves clinical, angiographic, and echocardiographic endpoints in a variety of clinical settings, including for patients with STEMI undergoing PCI,40-42 also resulting in optimal platelet aggregation in a large percentage of patients very early after PCI.43 The extent of platelet aggregation inhibition has been shown to correlate inversely with the risk of major adverse cardiac events after PCI.12
Choice of adjunctive study treatment
Thienopyridines have been shown to improve outcome in patents with non-ST elevation acute coronary syndrome, especially when given upstream and at a sufficiently high loading dose.44,45 The CLARITY-TIMI study showed the benefit of upstream therapy with clopidogrel in patients with STEMI as well.46 Moreover, the benefit of upstream therapy with clopidogrel extends to those patients with STEMI who undergo facilitated PCI (after fibrinolytic therapy).47 Further supporting the rationale for pretreatment with clopidogrel are results of a study in which patients receiving aspirin and a GP IIb/IIIa inhibitor who were pretreated with clopidogrel before coronary stenting experienced better outcomes at 30 days and 1 year than those receiving clopidogrel immediately after the procedure.48
The 600-mg loading dose of clopidogrel was chosen for the present study as the most effective dose both in conjunction with tirofiban, as well as for patients who will be receiving placebo. Results of prior studies suggest that a 600-mg loading dose of clopidogrel before PCI is safe and provides platelet inhibition greater than that provided by a 300-mg dose.49-54 After administration of the 600-mg dose, the full antiplatelet effect is achieved in two hours.53 For patients receiving placebo, further protection is afforded by possible use of tirofiban bailout after PCI.
The antiplatelet effect of clopidogrel, however, even when using a high loading dose (600 mg), is significantly lower than that of regular doses of GP IIb/IIIa inhibitors (Figure 3).49
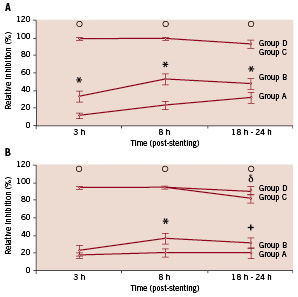
Figure 3. A, Platelet inhibition in response to 5 µmol/L adenosine diphosphate (ADP) after 4 treatment regimens. *P0.001, group A vs B; oP0.001, group C or D vs group A or B. B, Platelet inhibition in response to 20 µmol/L ADP after 4 treatment regimens. Group A received 300 mg clopidogrel; group B, 600 mg clopidogrel; group C, 300 mg clopidogrel + eptifibatide; and group D, 600 mg clopidogrel + eptifibatide. *P=0.09, group A vs B; +P=0.01, group A vs B; oP<0.001, groups C and D vs groups A and B; P=0.05, group C vs D. Reprinted with permission from Gurbel et al.48
It is suggested that even after high-dose thienopyridine therapy, platelet aggregation inhibition remains suboptimal and that adding a high-dose bolus of tirofiban in addition to aspirin and clopidogrel might result in superior platelet inhibition during PCI.49
Choice of primary endpoint
This study aims to determine the extent of residual ST-segment deviation (defined as percentage of patients with >3 mm deviation of ST segment) one hour after PCI initial patency of the infarct related vessel before PCI is not always related to improved outcome after PCI, as was shown in the ASSENT 4 trial55 where thrombolytic therapy given before transportation improved initial TIMI 3 flow, but did not improve outcome after PCI. Therefore we decided to chose residual ST deviation after PCI as the primary endpoint. The evaluation of ST-segment resolution has been shown to be a reliable method to analyse myocardial perfusion and infarct size in patients with STEMI treated by pharmacological or mechanical reperfusion.6,56-58 In fact, early resolution of ST-segment elevation correlates with myocardial salvage after reperfusion therapy.58 We previously reported that in patients with post-procedural TIMI 3 flow, ST-segment resolution significantly added prognostic information regarding long-term mortality.31 Results of more recent studies indicate that ST-segment resolution predicts both short- and long-term outcomes for patients who have undergone PCI.59-61
To further investigate and describe the effects of early GP IIb/IIIa receptor inhibition by tirofiban in this study, several substudies will be conducted, including evaluations at predetermined intervals of platelet function, myocardial salvage by single-photon emission computed tomography (SPECT), and as well as functional analysis and viability by magnetic resonance imaging (MRI). In this patient population, platelet activation is high. The goal of the platelet function substudy is to investigate whether the level of platelet inhibition as assessed with different point-of-care platelet function assays at two different time points (between angiography and PCI and at discharge) will correlate with clinical outcomes (periprocedural acute MI, death, TVR, stroke), as well as ST-segment resolution, TIMI flow, myocardial blush grade, and myocardial salvage (as assessed with SPECT). In a second substudy, myocardial salvage and infarct size will be assessed using Tc-99m sestamibi scintigraphy, as previously described.62,63 Patients will receive an intravenous injection of the radionuclide before angiography and PCI, followed by SPECT within 6 to 8 hours after the radionuclide injection and again 5-14 days after PCI. In the third substudy, MRI will be performed from 3-6 days after PCI and on follow-up after 4 months for a volumetric analysis of the left ventricle (size, volumes, and ejection fraction); to assess wall motion and thickening; and to assess myocardial viability (measurement of infarct size, microvascular obstruction zones, and infarct transmurality).
Platelet aggregation, MRI and SPECT substudies will only be performed in selected centres with special experience and equipment for high quality assessment of these parameters.
Summary
The On-TIME 2 trial, which will enrol 958 patients, evaluates the benefit of pre-hospital initiation of high-dose bolus tirofiban in patients with STEMI who undergo PCI. This placebo-controlled trial will provide important evidence regarding early initiation of a GP IIb/IIIa inhibitor, in combination with high-dose clopidogrel. If effective, this regimen may become part of standard care of STEMI.
Acknowledgements
The authors thank Vera Derks, Elizabeth V. Hillyer, DVM, ELS and Elaine See for editorial support. The study is designed and the conduct of the study is done by the investigators themselves. The support of Merck & Co Inc. for administrative expenses and supply of tirofiban and its placebo is gratefully acknowledged.
Trial registration
Current Controlled Trials ISRCTN 06195297
www.controlled-trials.com/ISRCTN06195297/06195297
Appendix
Trial committees
Executive Committee: A.W.J. van ‘t Hof, MD PhD, Isala klinieken, Zwolle, The Netherlands; J.M. ten Berg, MD PhD, St. Antonius Ziekenhuis, Nieuwegein, The Netherlands; C. Hamm Prof., MD PhD, Kerckhoff-Klinik GmbH, Bad Nauheim, Germany; S. Guptha, MD (non-voting), Merck&Co, Whitehouse Station, USA
Steering Committee: A.W.J. van ‘t Hof, MD PhD, Isala klinieken, Zwolle, The Netherlands; J.M. ten Berg, MD PhD, St. Antonius Ziekenhuis, Nieuwegein, The Netherlands; C. Hamm Prof., MD PhD, Kerckhoff-Klinik GmbH, Bad Nauheim, Germany; P. Stella, MD PhD, UMC, Utrecht, The Netherlands; L.F.M. van den Merckhof, MD, Scheper Ziekenhuis, Emmen, The Netherlands; A. Cieslinski Prof., MD PhD, Kliniki Akademii, Poznan, Poland; W. Kochmann, MD PhD, Swissmed Centrum Zdrowia S.A, Gdansk, Poland; J. Brachmann Prof., MD PhD, Klinikum Coburg GmbH, Coburg, Germany; T. Dill, MD PhD, Kerckhoff-Klinik GmbH, Bad Nauheim, Germany; G. Giannitsis, MD PhD, Universitätsklinik, Heidelberg, Germany; S. Guptha, MD (non-voting), Merck&Co, New Jersey, USA
Operational team: S. Guptha, MD, Merck&Co, New Jersey, USA; P. van Drooge, Merck, Sharp and Dohme B.V., Haarlem, The Netherlands; E. McAuly, Devlab, Hoddesdon, United Kingdom; K. Magerl, MSD, Haar, Germany; K. Lis, Merck&Co, New Jersey, USA; J. Klijn, Diagram B.V., Zwolle, The Netherlands
Data Safety Monitoring Committee. Chairman: A. Mosterd, MD PhD, Meander Medisch Centrum, Amersfoort, The Netherlands. Members: E. Boersma, MD PhD, Erasmus University, Rotterdam, The Netherlands; E. Eeckhout, MD PhD, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland. Unblinded statistician: E. Kolkman Msc, Diagram B.V., Zwolle, The Netherlands
CEC (Clinical Event Committee). Chairman: K. Dawkins, MD PhD, Wessex Cardiac Unit, Southampton University Hospital, Southampton, United Kingdom. Members: P. Widimsky Prof., MD PhD, Univerzita Karlova V, Prague, Czech Republic; H. White Prof., MD PhD, Auckland City Hospital, New Zealand
Contract Research Organisation (CRO): Diagram B.V., Van Nahuysplein 6, 8011 NB Zwolle, The Netherlands
Corelabs: ECG. Corelab: H. Suryapranata, MD PhD, Diagram B.V., Zwolle, The Netherlands; Angiographic Corelab: J.H.E. Dambrink, MD PhD, Diagram B.V., Zwolle, The Netherlands; Central Haematology Lab: G. Giannitsis, MD PhD, Universitätsklinik Heidelberg, Germany; MRI Corelab: T. Dill, MD PhD, Kerckhoff-Klinik GmbH, Bad Nauheim, Germany; Spect Corelab: J.M. ten Berg, MD PhD, St. Antonius Ziekenhuis, Nieuwegein, The Netherlands; Platelet Aggregation Substudy Corelab: W. van Werkum, MD, St. Antonius Ziekenhuis, Nieuwegein, The Netherlands.

