Introduction
In order to improve our understanding of late stent thrombosis and to assess the individual risk of a patient or lesion, there is clinical need to assess vascular healing after stenting in vivo. We will discuss the potential and limitations of optical coherence tomography (OCT) for imaging of drug eluting stents (DES).
OCT, a light based imaging modality1, has recently become available for intracoronary application2-4. Because of the shorter wavelength of infrared light compared to ultrasound, OCT has a ten-fold higher image resolution than conventional intravascular ultrasound (IVUS), 150 micron for IVUS as compared to 15 micron for OCT. This advantage renders OCT particularly useful for the assessment of coronary stents.
OCT observations in drug-eluting stents immediately after implantation
For the past two decades, IVUS has been used to assess the acute result following stenting, giving valuable information on stent expansion, strut apposition and signs of vessel trauma including dissections and tissue prolapse5,6. IVUS studies7,8 suggested that stent strut malapposition is a relatively uncommon finding, observed in approx 7% of cases, and that strut malapposition does not increase the risk of subsequent major adverse cardiac events. In contrast, OCT can visualise the complex coronary arterial wall structure after stenting in much greater detail9. As a result, OCT studies in the acute post stent setting10 have demonstrated a relatively high proportion of stent struts, not completely apposed to the vessel wall, even after high pressure post-dilatation. Furthermore, this phenomenon is particularly evident at regions of stent overlap11. Tanigawa et al12 examined a total of 6,402 struts from 23 patients (25 lesions) and found 9.1±7.4% of all struts in each lesion treated were malapposed. Univariate predictors of malapposition where: implantation of a sirolimus-eluting stent (SES), presence of overlapping stents, longer stent length and type C lesions. Likely mechanical explanations for malapposition of stent struts include increased strut thickness, closed cell design or acute stent recoil. The latter has been demonstrated in SES to be in the range of 15%, despite the use of high pressure balloon dilatation13.
While these findings are impressive and helpful for the improvement of future stent designs, today the clinical relevance and potentially long-term sequeale of malapposed struts as detected by OCT are currently unknown.
OCT observations in drug eluting stents at long-term follow-up
Unlike conventional stents, which develop circumferential coverage with an average thickness of 500 micron or more, well visualised with IVUS and angiography, drug-eluting stents delay and prevent the hyperplastic response so that the average late lumen loss for drug-eluting stents can be lower than 100 micron4 which means this thin layer of intimal thickening can be below the resolution of IVUS. Coronary angioscopy is able to visualise strut tissue coverage to a certain extent, but this highly specialised technique lacks the ability for quantification and the ability to assess thin amounts of neotinima. Hence, OCT is an attractive alternative, able to circumvent many of these limitations and, with its high-resolution, can precisely assess the in vivo tissue responses following stent implantation14. Specific applications of OCT relevant for late stent thrombosis are discussed below.
a) Visualisation and quantification of stent strut tissue coverage
OCT can reliably detect early and very thin layers of tissue coverage on stent struts (Figure 1-4). Several small studies have recently been published highlighting the application of OCT in the detection of stent tissue coverage at follow-up. Importantly, OCT permits the quantification of tissue coverage with high reliability15. Matsumoto et al16 studied 34 patients following sirolimus eluting stent (SES) implantation. The mean neointima thickness was 52.5 microns, and the prevalence of struts covered by thin neointima undetectable by IVUS was 64%. The average rate of neointima-covered struts in an individual SES was 89%. Nine SES (16%) showed full coverage by neointima, whereas the remaining stents had partially uncovered struts. Similarly, Takano et al17 studied 21 patients (4,516 struts), three months following SES implantation. Rates of exposed struts and exposed struts with malapposition were 15% and 6%, respectively. These were more frequent in patients with acute coronary syndrome (ACS) than in those with non-ACS (18% vs 13%, p <0.001; 8% vs 5%, p <0.005, respectively). The same group recently reported two year follow-up OCT findings18 with the thickness of neointimal tissue at 2-years being greater than that at 3-months (71±93 micron vs. 29±41 micron, respectively; p<0.001). Frequency of uncovered struts was found to be lower in the 2-year group compared to the 3-month group (5% vs. 15%, respectively; p<0.001). Conversely, prevalence of patients with uncovered struts did not differ between the 3-month and the 2-year follow-up study (95% vs. 81%, respectively) highlighting that uncovered struts continued to persist at long-term follow-up. Chen et al19 recently used OCT to image SES and bare metal stents (BMS) at different time points following implantation. Of the 10 SES and 13 BMS imaged, the authors identified a significantly higher number of incompletely apposed and uncovered stent struts in patients receiving SES compared to BMS.
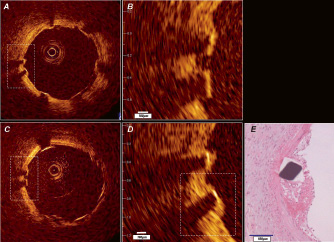
Figure 1. In vivo OCT (LightLabImaging™, Boston, MA, USA) in a porcine coronary artery. A) Baseline OCT immediately after stent implantation shows adequate stent expansion and apposition of the struts against the normal coronary wall. B) Magnification demonstrating the stent strut vessel wall interface. C) Follow-up investigation at five days. The stent struts are clearly visible and show thin, bright reflective tissue coverage in the magnification D) Histology E) confirms the presence of a thin neointimal layer.
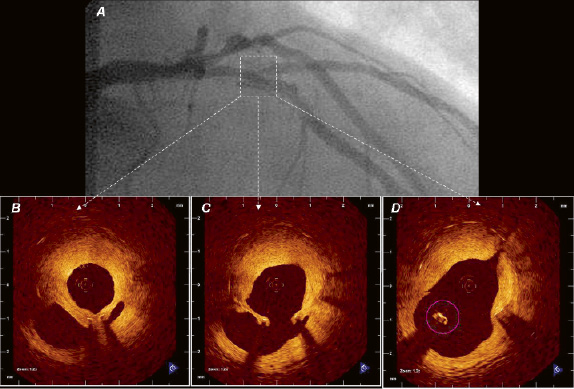
Figure 2. In vivo OCT (LightLabImaging™, Boston, MA, USA) in the LAD in a patient presenting with in-stent restenosis. A) The coronary angiogram shows a lumen narrowing within the stent that is covering the second diagonal branch. OCT visualises the complex coronary anatomy in great detail. The stent is covered by a thick neointima that shows a layered appearance with a bright, highly reflective luminal layer, an intermediate layer and a dark, signal poor layer surrounding the struts (B). The diagonal take off can be clearly seen (C) as well as a stent strut that is “floating” in the carina (D).
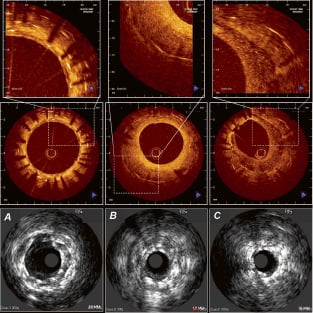
Figure 3. Demonstrates the information that can be gathered from IVUS (20Mhz, lower panels) and from OCT imaging (mid and upper panels, LightLabImaging™, Boston, MA, USA) in patients at follow-up after stent implantation. These are corresponding cross sections within a stent, imaged by both, OCT in the upper panels and by IVUS in the lower panels. The images represent the same spots within a coronary artery (A, B, C), and illustrates the different quality of information that can be obtained by OCT as compared to conventional grey scale IVUS. A) Three layers of stents can be seen. OCT is able to clearly visualise the individual stent struts, the neointimal layers separating the different stents and the very thin coverage of the most inner, luminal stent struts. B) a bright, eccentric and relatively thick neointimal layer can be seen C) an eccentric thick neointimal layer is visible, however, the structure of this neointima differs considerably from the example B) with a low-reflective and speckled appearance.
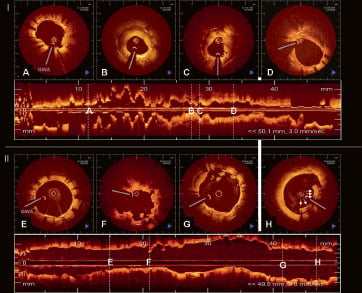
Figure 4. OCT (LightLabImaging™, Boston, MA, USA) findings in two patients presenting with late drug-eluting stent (DES) thrombosis. OCT was performed in both cases immediately after thrombus aspiration and reveals completely different morphologic findings, possible suggesting two different mechanisms for late stent thrombosis, focal restenosis and incomplete strut coverage. I) Patient with late stent thrombosis three months after DES implantation in the left circumflex artery. OCT reveals an adequately expanded stent, all struts are well apposed against the vessel wall. All struts show tissue coverage, which is more pronounced in the proximal portion of the stent (D) as compared to the distal stent portion (A). There is focal in-stent restenosis (B, C) with severe lumen narrowing (MLA 1.63 mm2). The neointima shows a layered appearance with a luminal bright, highly reflective layer, an intermediate layer and a dark, signal poor layer surrounding the struts. Remnants of the thrombus are focally seen focally as irregular mural structures, protruding into the lumen (A, C). II) Patient with very late stent thrombosis four years after DES implantation in the left anterior descending artery. OCT reveals an adequately expanded stent, however, there is incomplete stent strut apposition at the proximal stent edge with incomplete tissue coverage in 21% of struts. (E) The distal stent portion, shows a well expanded and apposed stent with thin tissue coverage by OCT. F) irregular lumen borders with intraluminal remnants of the thrombus. G) proximal stent portion showing a strut without visible tissue coverage in 12 o’clock position and thrombus fragments in the lumen. H) proximal stent edge with incomplete apposition of five stent struts (arrows) against the vessel wall. The distance to the vessel wall is 200 micron. OCT shows tissue around the stent struts (DD thrombus, neointima). GWA: guidewire artefact.
The results of these small observational studies are compatible with evidence from animal and human post-mortem series showing that DES cause impairment in arterial healing, some with suggested incomplete re-endothelialisation and persistence of fibrin(oid)20,21 possible triggering late stent thrombosis22.
However, OCT observations need to be interpreted with caution. OCT is limited by its resolution of 15 micron which is greater than the thickness of an individual layer of endothelial cells. Therefore, coverage that is not visible by OCT does not exclude the presence of an endothelial layer. Second, the presence of tissue coverage does not necessarily imply the presence of a functionally intact endothelium. Early experimental stent data showed that endothelial function can vary considerably and show evidence of damage when subjected to the Evan’s blue dye exclusion test23, even in the presence of a well structured neotintimal layer. In consequence, morphology should not be confused with function.
b) Assessment of structural details of tissue coverage
OCT also permits the characterisation of neointimal tissue in a qualitative way. This is a great advantage as such information has not been available in vivo until now. The limited resolution together with artefacts induced by metallic stent struts do not allow the characterisation of such details by IVUS. With OCT, neointimal tissue can show a variety of morphologies ranging from homogeneous, bright, uniform tissue to optically heterogeneous tissue or eccentric tissue of various thickness. Furthermore, structural details within the tissue can be observed such as intimal neovascularisation24 or a layered appearance, typically observed in restenotic regions25. Variations in the appearance of strut coverage can be seen within an individual patient, within an individual stent or within stents of different design.
OCT findings, such as dark, signal-poor halos around stent struts may reflect fibrin deposition and incomplete healing, as described in pathologic and animal experimental series20,21. However, there is paucity of data demonstrating directly the OCT appearance of different components in neointimal tissue as defined by histology. Post-mortem imaging of DES in human coronaries is difficult and might be limited by the fact that the optical tissue properties show variations with temperature and fixation26. Long-term animal OCT observations in DES are scarce.
c) Assessment of stent strut vessel wall interaction and strut apposition
The interest in the long-term stent strut vessel wall interaction is manifold and includes the assessment of the stability of the acute result, the visualisation of complex anatomy that is not accessible by angiography or IVUS and the clearer understanding of reasons for stent failures, when they do occur. The unique optical properties of OCT can also be applied to the study and evaluation of new stent designs including bioabsorbable stents. Morphologic changes of the absorbable, polylactic acid stent struts and the vessel wall during follow-up have been recently described and show the unique capabilities of this in vivo imaging modality27.
Stent strut malapposition remains an important consideration. Postulated causes for stent strut malapposition are various and include incomplete stent expansion, stent recoil or fracture, late outward vessel remodelling or the dissolution of thrombus that was compressed during PCI between the stent strut and the vessel wall. Regardless of the pathophysiologic mechanism, the major concern in stent malapposition remains in the assumption that areas of strut malapposition cause non-laminar and turbulent blood flow characteristics, which in turn can trigger platelet activation and thrombosis. Here, prospective, serial OCT observations immediately and at longer term follow-up after stenting may improve our understanding of these complex mechanisms and shed light on the likely clinical significance of this phenomenon.
Reasons for DES failure are poorly understood. With the reduction of in-stent hyperplasia, other mechanisms of restenosis due to mechanical stent failure have become apparent. Of the two established first generation DES, the sirolimus-eluting stent (Cordis, Johnson&Johnson, Miami, FL, USA) has been particularly linked to cases of stent fracture, likely as a result of its closed cell design compared with other DES employing an open cell system28. The higher imaging resolution of OCT compared to IVUS permits a detailed assessment in such cases, as demonstrated recently by Shite et al29.
Conclusion & future developments
OCT is a light-based diagnostic tool that allows in vivo imaging of the coronary artery wall in unparalleled detail30. OCT can reliably visualise very thin stent strut tissue coverage as early as five days after implantation, and permits for its qualitative and precise quantitative assessment. These unique capabilities favour OCT as the new golden standard for the evaluation of coronary stents.
Recent improvements in OCT technology, with frequency-domain OCT, will allow for a simple imaging procedure and offer the potential for large scale, prospective studies, indispensable to address vexing clinical questions such as the relationship of drug-eluting stent deployment, vascular healing, the true time course of endothelial stent coverage and late stent thrombosis. This may also better guide the optimal duration of dual anti-platelet therapy that currently remains unclear and rather empiric.

