Editors note
Last year we presented in EuroIntervention a short glimpse of the Technology Parade from the annual Innovations in Cardiovascular Interventions (ICI) meeting held in December 2007. Thanks to the positive feedback and the interest displayed from the interventional community we decided to invite ICI back again this year to highlight, in depth, the novel techniques and therapies as presented at the December 2008 meeting.
Preface
Innovative ideas are crucial not only in advancing medicine, but in better understanding how we practice today and in the future. We hope that these short summaries give you a taste, however brief, of emerging medical technologies and our future interventional landscape.
Biomedical technology is, in itself, one of the major engines enhancing progress in diagnostics and management of coronary artery disease. Since its conception the ICI meeting has focused on the interface between practice and technology, spotlighting clinical needs and the innovative technological solutions to them. It was out of this that “The Technology Parade” came into being, bringing together a professional public comprised of physicians, innovators and industry leaders to witness new technologies at their early stages of development. Here we will present 16 technologies spanning four major fields: imaging and diagnostics, heart failure, devices and simulation and navigational tools.
Imaging and diagnostics is a rapidly advancing and fascinating field where intense and on-going investigations attempt to determine the proper indications in relation to various non-invasive and invasive imaging modalities in use today. Non-invasive diagnostics based on physiological measurements using respiratory stress response are presented reflecting various modalities used to extract physiological signals that predict coronary artery disease. We also look at the user friendly Arinetta dedicated Cardiac CT that presents real-time data, minimising radiation exposure and focussing on the heart. The increase in percutaneous valve replacement has triggered new imaging modalities to precisely position these heart valves, one of these new systems, in development by Paieon Medical, is presented here as well.
Heart failure presents a challenge for patient management. New technologies introduced here touch on a variety of disciplines: pharmacologic, cell therapy, tissue engineering, various cardiac assist systems and possible devices. We look at chronic vagal nerve stimulation, proposed as a method to improve function and overall performance, preliminary animal findings are encouraging. The injection of a liquid biomaterial into an infarcted region that polymerises after delivery preventing remodelling, improving repair and limiting infarct-expansion is another proposed direction. A device based therapy for diastolic heart failure, a novel passive elastic device which transfers energy from systole to diastole, perhaps improving diastolic performance, is introduced. Finally, continued research into additional molecules to favourably modify the process of heart disease is represented by a new cardio-protective drug for myocardial preservation.
Device evolution remains an important part of our “Parade”. Coronary total occlusion continues to be a challenge due to moderate success rates in percutaneous revascularisation. One proposition – periodic balloon inflations and elongation resulting in forceful axial motion of an entrapped wire – is presented, while another, involving a tip dilation feature added to wire technology is also shown. In cutting-edge, stent technology, MGuard uses a polymer fine mesh covering a metal stent to prevent distal embolisation and we see an innovative biodegradable salicylate-based sirolimus-eluting stent. Cardiospec shock wave therapy is a new direction intended to enhance collateralisation and improve symptoms for patients with end-stage disease. Embolic stroke preventions is addressed using a novel method to filter out particles, while a booster technology to enhance blood flow to jeopardised limb circulation is perhaps an emerging answer for an old indication of peripheral limb salvage.
Simulation and navigation tools, previously the realms of dreams, are just now becoming reality. Critical new ideas that can improve education, training and practice are embodied in, for instance, a patient specific simulation where real-life data from patient specific anatomy is used to perform a proposed simulated procedure before you approach the actual intervention. A robotic navigational tool for coronary interventions represents the vibrant domain of robotics in surgery as well electrophysiological applications that are current practice worldwide.
I. The Arineta cardiac dedicated CT scanner
Background: Evaluation of coronary anatomy has traditionally relied on coronary angiography. Computed tomography (CT), on the other hand, has been traditionally used in radiology departments for other parts of the body. Advances in CT technology have made conventional CT fast enough to almost freeze the heart motion, thus providing a new imaging modality in cardiology.
Coronary CTA has a potential advantage over C arm angiography due to its non-interventional nature, inherent 3D capabilities and ability to visualise the vessel walls. Yet, with conventional CT technology, cardiac CT images suffer from compromised quality and sometimes non-diagnostic results. CT scan today involves relatively high radiation dose and the equipment is expensive, bulky and power consuming.
Technology: Arineta’s product under development is a high performance small size cardiovascular CT based on novel proprietary architecture, designed to provide uncompromised answers to cardiology needs. The Arineta CT provides coverage of 130 mm in a single 150 msec shot, allowing imaging of the whole heart within the diastolic phase of one cardiac cycle. Full image quality is achieved for the entire scan volume and image quality limitations of conventional CT are avoided.
Furthermore, the Arineta CT offers:
– 85% reduction of radiation dose compared to 64 slice spiral CT
– Reduction of contrast agent dose
– Small footprint, easy to operate in a minimal space
– Seamless integration in cardiology workflow
– Cost effective design, translated to lower end-user price
In addition to the basic protocol for imaging of the coronaries, the machine will support protocols for calcium scoring, dynamic imaging of the beating heart, myocardium perfusion and imaging of the aorta and peripheral vessels. The Arineta’s unique design might enable future routine applications such as:
– Screening of asymptomatic population
– Diagnosis of structural and functional cardiac defects
– Assessment of valve function
– Real-time “road mapping” in the cathlab
– Image-guided intervention
– Plaque mapping and classification
II. A novel non-invasive respiratory stress test indicates significant coronary artery disease
Introduction: Cardiac response to stressful respiratory manoeuvres has been shown to differ in patients with significant coronary artery disease (S-CAD).
Technology: The respiratory stress response (RSR), an index derived from spectral analysis of the finger photoplethysmography (PPG), is intended to quantify the oscillations of the PPG signal during 70-sec of deep paced breathing at a rate of 6 breaths per minute (0.1Hz).
Objectives: To evaluate RSR as an indicator of S-CAD.
Methods: Patients referred for coronary angiography at the Washington Hospital Center performed the RSR test prior to the procedure. RSR (%)= respiratory peak area at 0.1Hz divided by area of other regions in the spectrum. The coronary angiograms were analyzed by quantitative coronary angiography (QCA), blindly to the RSR results. S-CAD was defined as luminal stenosis > 70% of at least one coronary artery with reference diameter > 2 mm.
Results: A valid RSR was obtained in 150/153 (98%) pts, mean age= 58.7+10.6 years, 67% men. S-CAD was found in 36 (24%) pts. These pts had significantly lower RSR compared to pts without S-CAD, (6.7+5.1 vs. 17.4+10.6, p<0.001, respectively). RSR <10.2%, yielded sensitivity of 86% (95% CI= 70%-95%), specificity of 81% (95% CI=72%-88%), for detection of S-CAD. Multivariate logistic regression analysis, adjusted to known CAD risk factors found RSR to be a strong independent indicator of significant CAD (OR=41.2, 95% CI=12.2-139.3, p<0.001)
Conclusions: The novel RSR is a simple, non-invasive bedside or office based test to detect S-CAD.
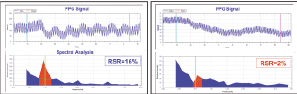
Reduced PPG signal oscillations and hence, respiratory stress response in significant CAD.
III. CardiOp-THV - angiography-based system for trans-catheter heart valve (THV) procedure planning and real-time positioning
Introduction: Senile calcific aortic disease resulting in either stenosis, regurgitation, or a mixture of both, is the most common aortic valvular disease. While the surgical replacement of the severely calcified stenotic valve is considered the primary treatment, it entails the risks and morbidity associated with cardiopulmonary bypass and median sternotomy. As a result, many patients with severe aortic stenosis (AS) do not undergo surgery due to the excessive risk and multiple comorbidities. The development of the percutaneous aortic valve replacement (PAVR) and the promising results from the preliminary experience placed this technology as an alternative therapy for inoperable patients.
The Edwards SAPIEN transcatheter heart valve (THV) was first deployed in human in 2002. Since then, more than 1,500 patients were treated by this device. The accumulated experience showed that the non-frequent procedural complications, paravalvular regurgitation, stent embolisation, and coronary obstruction are mostly related to device inappropriate positioning, mal-sized prosthesis and inadequate expansion of the stent. In order to overcome these obstacles, Paieon Medical is developing novel software aimed for optimizing the THV procedure.
The new technology: The CardiOp-THV, is an angiography-based system based on three stages. The first, procedure planning, is comprised of selection of perpendicular projection to the aortic annulus, and the appropriate stent size. The second stage is real time positioning of the THV and the final stage is the post-deployment analysis; i.e., measurement of the THV’s internal and external diameters following expansion.
The prototype was released in August 2008. It is installed and operated in five leading medical centres worldwide. The preliminary experience has demonstrated promising results for optimal projection, post-deployment analysis and real time positioning. The angiographic based sizing methodology is still under investigation resulting in encouraging accumulated data.
Summary: The CardiOp-THV is a novel application which addresses major procedural obstacles and challenges. The consistent accurate prototype results, will lead to the first generation release within few months.
IV. Chronic vagus nerve stimulation, a new treatment for congestive heart failure (CHF)
Background: BioControl Medical, a clinical stage, private medical technology company, develops implantable neurostimulation systems for the treatment of autonomic dysfunction.
Technology: The company’s most advanced product, the CardioFit™ system, is intended as a treatment for chronic heart failure either as an independent therapy or in combination with other existing, approved therapies. According to the AHA, HF afflicts 5.2 million Americans with 550,000 new diagnoses and more than 275,000 deaths annually. The study on HF awareness and perception in Europe estimates that nearly 14 million Europeans currently suffer from HF. The disease often leads to long hospitalisations, and death. In addition, the costs for treatment and hospitalisation in the U.S. have reached 35 billion dollars annually. CardioFit™ uses a pulse generator and lead system with control algorithms that allow for fine adjustment of parasympathetic input to the heart while minimising side effects of vagal nerve stimulation.
Clinical testing: To date, BioControl has completed several pre-clinical studies and a clinical pilot study. Preclinical studies demonstrated that cardiac dimensions and cardiac function were markedly improved in CardioFit™ treated animals, while sham treated controls continued to worsen. Initial data from the European clinical pilot study show that CardioFit™ implantation is feasible, safe and well tolerated. Preliminary clinical data also suggests efficacy. The company completed enrollment for the pilot study towards the end of 2008. Six month follow-up data of 20 subjects shows that 14 subjects improved their NYHA class, and six remained stable. Significant improvement was also found in LVEF, quality of life and exercise capacity. In December 2008, BioControl received a CE Mark certification for CardioFit™ from KEMA, a Dutch notified body, in accordance with the European Active Implantable Medical Device Directive.
Future perspective: BioControl is now planning an international, multicentre, pivotal study of CardioFit™ which will begin at sites in the U.S. following FDA approval of the protocol.
V. Reverse remodeling after myocardial infarction with intracoronary injection of BioLineRx’s BL-1040 myocardial implant
Background: Cessation or reversal of progressive left ventricular remodeling and dysfunction are major therapeutic goals after myocardial infarction (MI). BioLineRx’s BL-1040 represents a breakthrough approach to support cardiac tissue damaged as a result of acute MI, improving cardiac function and survival.
Technology: BL-1040, a novel myocardial implant, is a resorbable liquid polymer that is administered via the coronary artery during catheterisation. The liquid polymerises within the infarcted cardiac tissue and forms a “scaffold” that enhances the mechanical strength of the heart muscle during recovery and repair, and is excreted naturally from the body within six weeks after injection.
Clinical and preclinical testing: A Phase I/II clinical trial designed to assess the safety and preliminary efficacy of BL-1040 in up to 30 patients is currently taking place. Interim results of the first five patients whom have already been safely treated are expected during Q2, 2009, and final results of the pilot Phase I/II study are expected in Q3, 2009. BL-1040 was shown, in pre-clinical trials on various species, to improve survival and be safe and effective in preventing deterioration of the myocardium by reducing left ventricle dilatation and eventual heart failure.
Conclusions: Intracoronary injection of BL-1040 myocardial implant is feasible and effective. Our findings suggest a new percutaneous intervention to improve infarct repair and prevent adverse remodeling after MI.
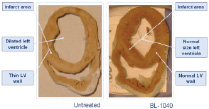
Figure. BL-1040 protects heart tissue from damage after myocardial infarction (MI). The volume of the left ventricle of a pig 2 month following an MI is pathologically increased due to thinning of the heart’s wall (left). Treatment with BL-1040 prevents the pathological remodeling of the heart (right).
VI. Efficacy assessment of a new device-based approach for treating diastolic heart failure
Introduction: Diastolic heart failure (DHF) accounts for over 40% of HF cases. Treatment of DHF patients is disappointing. We used a mathematical model, in vitro simulator and in vivo studies to evaluate the efficacy of a new approach for treating DHF, directed towards enhancing left ventricular (LV) filling, utilising an elastic device which gains its energy during systole and releases it in a recoiling force at diastole.
Methods: The device is screwed to the epimyocardium of the left ventricle free wall.
The device was evaluated in vitro, utilising a ventricular model: a constant volume of fluid is delivered by a pump inflating a LV model. When inflation is complete, the pump evacuates the fluid from the model until it returns to its initial volume. Pressures were measured with and without the ImCardia device.
In vivo: Hypertrophy was induced in mini-pigs by bilateral renal wrapping (Page model) which resulted in severe hypertension with consequent LV hypertrophy. The device was then implanted on the beating hypertrophied heart of two animals. One other hypertrophied heart served as a control (without device). Echo 2D strain analysis was done, evaluating the long term efficacy.
Results: In vitro studies showed a reduction in modelled end diastolic pressure with the device.
Two D strain analysis revealed device related global diastolic changes: increased peak diastolic strain rate, increased strain rate E/S ratio and increase in early reverse rotation (from time to peak angle to time to peak + 50 ms).
Conclusion: This study demonstrates that a passive elastic device, which transfers energy from systole to diastole, may improve diastolic performance.
VII. TVP1022 - a novel cardioprotective drug, for the treatment of acute coronary syndrome (ACS) and congestive heart failure (CHF)
Background: TVP1022 is the optical S-isomer of the newly developed, FDA-approved, anti-Parkinson drug Rasagiline, which was recently described as “a disease modifying drug” due to its anti-apoptotic and neuroprotective effects. Since: (1) TVP1022 is neuroprotective, and (2) cardiomyocytes and neurons share many similar features, we hypothesised that TVP1022 (which has minimal MAO inhibition), is also cardioprotective.
Materials and methods: The therapeutic efficacy of TVP1022 was demonstrated in 2 animal models of cardiac pathologies:
1. Acute coronary syndrome (ACS) induced by left anterior descending (LAD) artery occlusion in rats.
2. Congestive heart failure (CHF) induced by volume-overload model created by placement of a fistula between the abdominal aorta and the inferior vena cava in rats.
Results
1. ACS: Infarct size was reduced by 50% in the TVP1022-treated group.
2. CHF: Pre-treatment of rats with TVP1022 attenuated the decrease in fractional shortening, the remodeling, the hypertrophic response, the increase in BNP levels and the fibrosis.
Conclusion: The ability of TVP1022 to attenuate cardiac damage induced by different pathological insults, by means of its unique mechanisms of action, being anti-apoptotic, anti-ischaemic as well as anti-hypertrophic, render this molecule a potential cardio-protective drug for ACS and CHF patients.
VIII. Novel catheter technology for total occlusion crossing in peripheral vasculature: first human use
Background: Chronic total occlusion (CTO) is defined as a complete or nearly complete arterial occlusion for more than 30 days. The treatment of peripheral occlusions aims to improve clinical symptoms and decrease the need for vascular bypass surgery. CTOs are typically composed of hard fibrotic and sometimes calcified proximal and distal caps. They are refractory to conventional floppy guidewires with which insufficient axial force can be applied to achieve sufficient advancement. Novel catheter technology has recently been developed to facilitate recanalisation of many previously intractable CTOs.
Technology: The ENABLER-P CTO Crossing System has been developed to facilitate the intraluminal advancement of conventional guidewires beyond highly stenosed lesions, including CTO in the peripheral vasculature. The ENABLER-P employs over-the-wire balloon catheter technology in a novel configuration to aid in catheter advancement. Balloon inflation anchors the catheter against the vessel wall. Additional inflation of the balloon results in guidewire gripping and balloon elongation, and axial guidewire advancement. An external pressure control unit provides the physician with control over the inflation and deflation of the balloon and the guidewire advancement.
Clinical use: To date, a total of five cases were performed. The system was successfully used in all five procedures and successfully crossed the lesion in four procedures (guidewire advancement in a false lumen occurred in one case) with an average crossing time of 2.5 min, which potentially reduce procedure time, and radiation exposure. No serious adverse events were reported.
Conclusions: Based on the experience in five patients, it appears that this novel catheter technology is a promising and safe solution for CTO crossing.
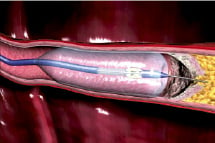
Figure. ENABLER-P Technology. Balloon is anchored against vessel wall, gripping the guidewire and pushing it through the occlusion.
IX. The new 0.014” CiTop™ guidewire for the treatment of chronic total occlusions in peripheral arteries: results of the first-in-man randomised clinical study
Background: Despite the development of novel interventional devices, chronic total occlusion (CTO) still remains a challenging problem in endovascular peripheral intervention, mostly due to inability to cross the lesion with the guidewire. We describe herein the first in man randomised study comparing the new CiTop™ guidewire to conventional wires in peripheral CTOs.
Technology: The CiTop™ guidewire is a 0.014” guidewire which combines conventional guidewire navigational properties with a tip dilation feature to allow its advancement through difficult occlusions
Clinical studies: Nineteen patients with 24 peripheral CTOs were randomly assigned to the CiTop™ guidewire or to conventional wires as a first wiring attempt to penetrate a total occlusion. Study endpoint was a successful crossing of CTO in a distal true lumen without a device-related adverse event. The CiTop™ guidewire successfully crossed the CTO in 13 out of 14 occlusions (92.3%), whereas a standard wire was able to cross in four out of 10 occlusions (40%). From this group of patients, five CTOs were crossed over to CiTop™ guidewire attempts where successful crossing was observed in four cases (80%). No technical problems or adverse events associated with the CiTop ™ guidewire usage were noted.
Conclusion: Our preliminary results demonstrate that the new 0.014” CiTop™ guidewire is safe and efficacious for the treatment of peripheral CTOs. The CiTop™ guidewire may serve as an excellent first choice wire in attempting CTO recanalisation.
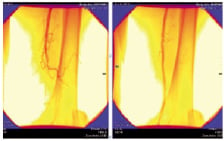
Figure. A successful case recanalised by CiTop guidewire.
X. MGuard - net protective stent
Background: Coronary embolisation is one of the most problematic complications of PCI, leading at times to catastrophic results. Patients at risk for this complication are those with ACS or AMI with a large thrombus, and patients undergoing intervention of old degenerative vein grafts.
0MGuard represents an innovative approach to embolic showers by merging a stent and an embolic protection into one device. It provides a high degree of embolic protection during and post-procedure, while maintaining the same deliverability as an ordinary stent. MGuard is an ultra-thin polymer mesh sleeve attached to the external stent surface and designed to reduce embolisation during coronary interventions. MGuard received CE mark in November 2007.
Preliminary preclinical testing: Coronary porcine studies demonstrated good feasibility and absence of safety issues like device thrombosis or animal morbidity. Histology data from the stented segments suggest that MGuard yielded acceptable inflammatory response with no medical necrosis, mineralisation, neovascularisation or granuloma response. Low Schwartz injury score, absence of fibrin score and exceptionally good endothelisation were noticed. The extent of intimal hyperplasia was acceptable.
Pilot clinical experience: This FIM study was conducted in Germany on 41 patients with native coronaries (44%) or SVG (56%) lesions. Protocol included six months angiographic follow-up and primary endpoint was one month MACE. Per protocol 6 months analysis for the total population showed 0% cardiac death, 12.1% TLR, 6.1% MI and late loss of 0.63mm. The 30 days MACE was 3.0% in general and 4.3% in the SVG group. Summary and conclusions: MGuard is a promising technology for coping with acute embolic complications, while maintaining procedure simplicity and safety.
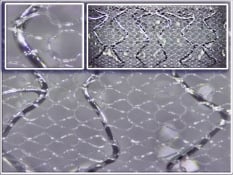
Figure. Miscellaneous myocardial interventions.
XI. Novel fully biodegradable salicylate-based sirolimus-eluting stent
Background: Recent advances in bioabsorbable stent technology have contributed to awakened interest in their role as alternatives to current metallic drug-eluting stents. We sought to evaluate a novel, fully bioabsorbable sirolimus-eluting stent (SES) synthesised entirely from salicylic-acid polymer, in a clinically relevant animal model.
Methods: Bioabsorbable balloon-expandable stents (n=32) were implanted in pig coronaries using quantitative coronary angiography (QCA) and intravascular ultrasound (IVUS) to optimise stent apposition. Dose density of sirolimus was 8.3 μg/mm stent length with in vitro studies demonstrating elution over 30 days and complete stent degradation in 9-12 months. Animals underwent QCA and IVUS restudy and were terminated at 7, 14, 30, 90, and 180 days for histologic assessment. Optical coherence tomography (OCT) was also performed for the 90- and 180-days samples.
Results: All stents were deployed successfully without notable mechanical difficulties. No edge dissection or vasospasm was observed during implant. No stent migration was observed at any time. Angiographic diameter stenosis (DS) was 20±16%, 24±4%, and 23±17%, at one, three and six months, respectively. In parallel, IVUS showed good apposition of the stent to the vessel wall with DS of 21±9%, 25±7%, and 18±3%; and area stenosis (AS) of 35±13%, 33±7%, and 32±4% at one, three and six months, respectively. OCT demonstrated good apposition of the stent with DS of 28±7% and 20±6%, and AS of 37±10% and 33±13% at three and six months, respectively. OCT showed reduction of stent thickness by 23% from three to six months. Histological analysis confirmed these in vivo findings and revealed a favourable healing process of absorbable stent incorporation into the arterial wall, without excessive thrombotic or inflammatory reactions.
Conclusions: This study shows favourable vascular compatibility and efficacy for a novel fully bioabsorbable salicylate-based SES. This device has good mechanical performance during deployment and stays well-apposed to the vessel wall at long term follow-up. These initial results are highly encouraging and support progress into more extensive preclinical studies as well as early clinical testing.

Figure. Fully bioabsorbable sirolimus-eluting stent.
XII. New treatment option for endstage CAD patients using extracorporeal myocardial revascularisation therapy (ESMR) using low intensity focused shockwaves
Gil Hakim
Background: Treating myocardial ischaemia symptoms in endstage CAD patients can be a challenge. The number of patients having this condition increases rapidly due to improved revascularisation techniques. In vitro and animal data show an increase of angiogenic factors after treatment of low intensity shock waves. The following demonstrates a new non-invasive therapy using focused, low intensity shock waves to induce local angiogenesis at myocardial ischaemic areas not treatable by conventional methods.
Technology: Low intensity shockwaves are generated extracorporeally using an electro-hydraulic effect. The treatment is performed using a shock wave generator system (Cardiospec, Medispec, Germantown, MD, USA) designed to address the clinical-anatomical requirements of the chest cavity. A cardiac ultrasound imaging system is used to locate the treatment area and guide the shock waves into it. Shock waves are then delivered via a special applicator though the anatomical acoustic window of the treatment area and under E.C.G. R-wave gating to avoid arrhythmias.
Clinical experience: Studies from 12 different medical centres, with 219 patients with refractory anginadue to three vessel disease or total occlusion of one epicardial vessel were treated. All had to have proven reversible ischaemia in at least one myocardial segment using rest/stress SPECT mapping. About 3 x 1500 impulses were applied to ischaemic areas using energy level of 0.09 mJ/mm2.
Results: Clinical results have shown significant symptomatic improvement in CCS class and exercise tolerance and significant improvement in perfusion by SPECT. Therapy was well tolerated by all patients. No side effects were present (arrhythmias, cardiac enzyme rise and new wall motion abnormality).
Conclusion: These data show that extracorporeal myocardial revascularisation therapy using low intensity focused shock waves may be an alternative, non-invasivemethod in the treatment of myocardial ischaemia in endstage CAD patients.

Figure. CCS class improvement up to six months.
XIII. Proactive prevention of embolic stroke – a new frontier
Objective: Stroke from particulate cardioemboli remains a major clinical problem. The evolution of transcatheter procedures as well as minimally invasive surgery for valve implantation, valve repair, closure of LAA etc.; have in fact increased the risk for embolic stroke. Embolic stroke remains a major etiology for mortality and the foremost cause for disability.
We have developed an aortic arch filter preventing embolisation from the heart and aorta to the brain, through its arterial supply. The filter is made of fine nitinol, with pore size of ~200 µm (aortic embolic protection device – AEPD). AEPD can be inserted transcatheter or directly into the ascending aorta. The AEPD diverts downstream all clots and debris as well as air bubbles. It has a designated delivery and removal catheter system.
AEPD is coated with ultrathin layer of hydrophilic lubricant polymer and heparin.
Methods: AEPD was tested in preclinical studies, including simulated pulsatile flow duplicator, and in nine series of sheep / pig tests. Efficacy was assessed in simulated pulsatile flow model.
Results: AEDP shows ease of introduction and removal, stability in location, lack of vascular damage and X-ray visibility. Efficacy is >90%.
Conclusion: AEPD is a proprietary aortic embolic protection device. It can provide proactive safety from particulate embolic stroke. Clinical studies are required to prove the safety of the technology.
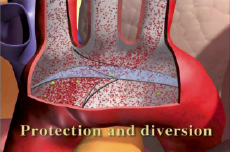
Figure. AEPD1 in the aortic arch.
XIV. A novel vascular booster device for critical limb ischaemia: feasibility and safety studies
Background: Critical limb ischaemia (CLI) is a result of severe decrease in distal blood flow (BF) and arterial blood pressure (ABP) caused by severe occlusive disease. Major limb amputation may ensue in patients who are not amenable to bypass surgery or angioplasty.
Technology: We devised an implantable peri-arterial ECG gated double balloon booster system that is capable of increasing downstream pressures by boosting BF in the presence of a semi-rigid sleeve restrainer and a non-return valve mechanism (PerAssist Ltd, Misgav, Israel). We first studied the effects of this system in an acute hind limb ischaemia model. Also safety of a 28 day implantation were studied.
Preclinical studies: Six anesthetised sheep were surgically fitted with the booster system on the iliac arteries. A controlled stenosis was produced in one of the femoral arteries by a screw occluder. Proximal and distal ABP were recorded by standard pressure transducers and BF by a transit time flow meter (Transonic, Ithaca, NY, USA). One way boosting was achieved by an external ECG gated rapid fluid pump and continued for three hours. The arteries were then harvested for histological studies.
Chronic safety studies were done in six sheep with boosters implanted around a carotid artery. The boosters were activated in conscious animals three hours daily for up to 28 days. Clinical, angiographic and histological studies were done. In the acute studies after 3 hours of boosting no visible arterial wall injury and no intra-arterial thrombosis or embolisation and no mortality were recorded. In control arteries without stenosis, the changes in either distal ABP or BF before or after boosting were non-significant (p>0.05). In stenosed arteries the distal mean ABP decreased by 22±6 mmHg (p<0.05) and distal BF by 51±7 ml/min (p<0.05). A 20-40% ECG gated time delay in upstream boosting resulted in a marked increase in both distal mean ABP (185+5% p<0.01) and downstream BF (88±21%, p<0.01). Histology studies of the arteries showed mild to moderate intimal injury in 3/6 suitable arteries, with no thrombosis and no significant medial and adventitial damage.
In the chronic studies in conscious animals no pain was observed upon activation of the booster. One system showed gross infection due to wound dehiscence. Angiography showed normal flow in the studied arteries. Histology showed only minimal changes after 28 days of boosting, mainly in the adventitia.
Conclusions: This study proves that the peri-arterial booster system is capable of significantly increasing distal ABP and BF across an arterial stenosis. This effect is equivalent to increasing ABI in CLI patients. Chronic safety studies showed no significant arterial damage due to the booster. Human acute feasibility studies are planned before chronic clinical studies in CLI patients are contemplated.
XV. Patient specific simulation enabling hands-on rehearsal of carotid stenting
Introduction: Endovascular procedures like carotid stenting can pose challenges even to experienced interventionists. Tortuous anatomy, complex lesion morphology, and difficult device access can increase procedure time, fluoroscopy exposure, contrast use, and complications.
Technology: Simbionix PROcedure Rehearsal Studio™ simulates the interventional environment and allows rehearsal of a complete endovascular procedure on a virtual model of the patient’s specific anatomy. Loading the patient’s CT scan directly into the system generates a digital 3D model of the patient’s clinically relevant anatomy. It generates a simulated interventional suit environment that provides the impression of a real procedure on a real patient. This includes display of the c-arm, visual presentation of fluoroscopic images, and reliable tactile feedback of the movement and function of intravascular devices.
Preliminary testing: Initial experience with the system demonstrated ease-of-use, and accurate vascular simulation. Rehearsal of complex cases of carotid stenting gave the operators the opportunity to practice the specific anatomy of the patient and to select the appropriate devices for the specific anatomy of the patient, in advance of the actual intervention. The actual interventional procedures were eventually easier and faster.
Conclusion: Using patient specific simulation with the Simbionix PROcedure Rehearsal Studio™ helps with planning of procedure and device selection, leading to use of less contrast and radiation, and shorter procedure duration. These benefit the patient with increased success and lower complication rates. Virtual rehearsal, derived from patient-specific simulation may increase the demand for the placement of simulators in larger number of institutions.
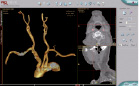
Figure. Simbionix PROcedure Rehearsal Studio™.
XVI. The Corindus Vascular Robotics System
The CorPath™ Vascular Robotics System developed by Corindus enables the cardiologist to perform coronary procedures from an interventional cockpit inside the procedure room.
The remote workstation removes the physician from the radiation field and reduces the risk of exposure to harmful X-ray radiation. The ergonomic design may also reduce cervical and spinal problems created from wearing lead aprons.
The vascular robotic system consists of an articulating robotic controller and sterile cassette that are mounted on the bed rail of the procedure table, and a physician control console.
The open architecture system manipulates standard cathlab devices using physician guidance, allowing precise placement of guidewires and balloon/stent delivery systems. Discrete device movements enable the physician to accurately deliver a device while avoiding partial obstruction of a branch artery. A key advantage of the CorPath System is the ability to fixate the guidewire or device during the procedure to avoid accidental wire or device movement during the intervention.
The first generation CorPath System was successfully tested in 30 patients in 2007. The study showed the feasibility of performing robotic PCI in humans. At 30 day follow-up there were no incidences of MACE reported. MACE was recorded in one non-target vessel.
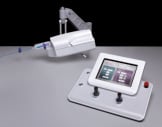
Figure. The Corindus Vascular Robotics System.

