Abstract
Aims: To investigate the endothelium independent coronary smooth muscle vasomotor function four months after implantation of magnesium alloy absorbable metal stents (AMS) as part of the Progress-AMS clinical trial (n=5), compared with a control group of patients implanted with permanent metal stents (PMS) (n=10) undergoing follow-up angiography, but who were free from angiographic restenosis.
Methods and results: Quantitative coronary angiogram (QCA) using an automated edge detection system was performed before and after the administration of 2mg intracoronary isosorbide dinitrate (ISDN). The vessel diameter was measured at 0.2 mm intervals throughout the stented segments and a 1 cm proximal reference segment. The cross sectional area (CSA) was calculated before and after the ISDN, averaged and the percentage change measured.
Reference segments demonstrated preserved vasomotor function in all cases: +13.28% (AMS) versus +17.15% (PMS), p=0.39. The mean percentage increase in CSA for the stented segment was +6.78% for the AMS versus –1.30% for PMS, p= 0.003.
Conclusion: These data demonstrate that four months after AMS implantation vasomotor function in reference segments is no different to that observed with PMS. However in contrast to PMS, within the AMS-stented segments there is demonstrable vasodilatation. These observations may have important implications for future stent design.
Introduction
Coronary stent placement was introduced with the aim of mitigating the major complications of percutaneous transluminal coronary balloon angioplasty, which are namely abrupt vessel closure, and restenosis.1 Intracoronary stenting however created problems of its own, most notably in-stent restenosis due to neointimal hyperplasia and stent thrombosis.2,3 The first generation of durable polymer drug eluting stents (DES) were very effective at preventing restenosis4,5 however they do not prevent, and may even increase, the risk of stent thrombosis which may be incremental6 and which is less common than restenosis, but carries a grave prognosis7.
Further detrimental characteristics of permanent metallic stent (PMS) implantation include ongoing physical irritation, chronic inflammation,8 prolonged myo-intimal proliferation9, distal endothelial dysfunction10,11 and in addition, by converting the stented segments into a “rigid tube”, with endovascular metallic scaffolding, these permanent prostheses prevent normal vasomotion within the stented segment. PMS implantation may also compromise later surgical revascularisation, and causes degradation of non-invasive imaging of the stented segment.
Whilst the majority of stent trials focus on early outcome, latterly a number of studies have suggested that there may be important longer term concerns about the clinical outcome with DES and indeed the role of stenting for coronary artery disease has been called into question12.
It seems therefore that PMS deployment, whilst being an effective short term revascularisation strategy, in spite of significant developmental achievements has important limitations. Furthermore, the trial proven benefits of the technology are early and thus the longer term function and safety of stents is unclear.
The Progress-AMS (Clinical Performance and angiographic Results of Coronary Stenting with Absorbable Metal Stents) clinical trial was a non-randomised, multicentre trial assessing the safety and efficacy of an absorbable magnesium alloy coronary stent. The methods and results have been published13. The study demonstrated safety for the technology although there was a significant rate of restenosis driven principally by reduction of the stent diameter during corrosion, probably because the corrosion/alloy characteristics did not give sufficient radial strength for long enough to allow arterial healing. The purpose of the present study was to investigate the possibility of the return of coronary vaso-reactivity within the segments stented with the AMS following stent absorption.
Materials and methods
In the present analysis we studied eight patients who underwent percutaneous coronary intervention (PCI) with AMS (Biotronik, Berlin, Germany) at The Royal Brompton and Harefield NHS Trust Hospitals as part of the PROGRESS-AMS trial. In brief, the patients were eligible for the trial if they had symptomatic ischaemic heart disease or silent ischaemia and a discrete de novo lesion in a coronary artery with reference diameter between 3.0 and 3.5 mm and lesion length 13mm or less. The stents were laser cut from magnesium alloy (>90% magnesium, elastic recoil: < 8%, collapse pressure: > 0.5 bar, foreshortening: < 5%) with corrosion kinetics designed to give resorption by four months. In the main study 63 patients were recruited, after obtaining informed written consent, and systematically investigated with follow-up coronary angiography at four months following the implant.
Follow-up coronary angiography was performed using a digital X-ray system (GE INOVA 2000™ GE Medical Systems Ltd, Chalfont St Giles, United Kingdom). Two orthogonal views were acquired before and after administration of 2 mg intracoronary isosorbide dinitrate. Quantitative coronary angiography (QCA) analysis was performed off-line. Two orthogonal views were analysed by two independent observers using computerised edge detection software (GE Medical Systems Ltd, Chalfont St Giles, United Kingdom). The diameter of the vessel was measured at 0.2 mm intervals throughout the entire length of the stented segment. The initial implant angiogram and stent deployment acquisitions were used to accurately gauge the location of the formerly stented segment. Additionally a 1 cm long segment, proximal to the stent was identified and measured as a reference. From the diameter the cross sectional area (CSA) of the formerly stented and reference segments were calculated (=r2) and averaged before and after the administration of intracoronary nitrate.
A control group of 10 patients who had received PMS in the previous year, underg hy for clinical indications, who were free from angiographically visible in-stent restenosis, were studied using the same technique. Comparisons between mean CSAs were made using the Mann-Whitney statistic.
Results
The clinical and procedural characteristics for the two groups are shown in Table 1. Three of the eight patients developed significant in-stent restenosis requiring repeated revascularisation. Five patients who were studied were free from significant restenosis. The patient level cross sectional area data for both cohorts before and after vasodilatation is shown in Table 2. The reference segments demonstrated preserved vasomotion function in all cases; +13.28% (AMS) versus +17.15% (PMS), p=0.39. The mean percentage increase in CSA for the stented segment was +6.78% for AMS versus -1.30% for PMS p=0.003 (Figure 1, 2).
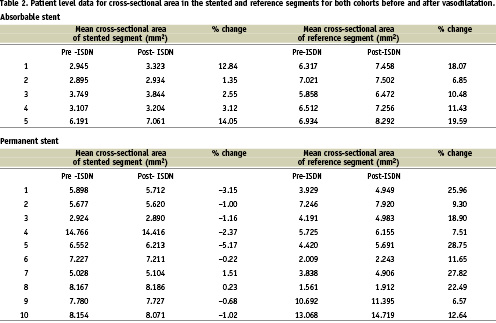
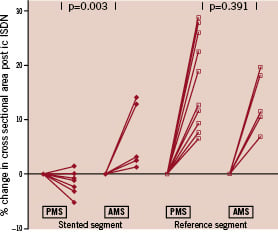
Figure 1. Graph illustrating the change in mean cross-sectional area pre- and post-intracoronary isosorbide dinitrate in the previously stented segment of the bioabsorbable magnesium stent (AMS) patients and a control group of permanent metal stent (PMS) patients with respect to a reference segment.
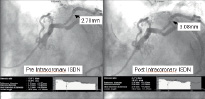
Figure 2. Representative coronary angiogram of a PROGRESS AMS patient at four months following absorbable magnesium stent implantation pre- and post-intracoronary isosorbide dinitrate injection indicating vasodilatation within the previously stented segment.
Discussion and conclusions
Intracoronary stent technology has developed rapidly resulting in excellent acute performance. Until recently, however, the vast majority of research has focussed upon strategies to overcome in-stent restenosis and relatively short-term outcomes. There is now a growing awareness that longer-term outcomes and stent thrombosis need to be aggressively studied; and in particular, that aggressive anti-restenotic technologies may in fact yield an unfavourable long-term result. Given the excellent early performance of modern stents, it is not surprising that the PROGRESS-AMS trial failed to deliver reduced levels of restenosis compared with contemporary technology, however it did provide data supporting proof of principle and safety for bioabsorbable technology.
It seems likely that the focus on long-term biocompatibility and safety will intensify and perhaps the best long-term strategy is to have no remaining permanent stent prosthesis. Among the potential benefits of this strategy is the potential for improved vessel healing. Our data suggest that there may be the potential for active vasomotion in AMS stented segments which is not seen in PMS stented segments.
The present analysis has a number of important limitations, including sample size, and a limited mechanistic analysis of the vasomotor response: for example interrogating endothelium dependant vasomotion was restricted due to established study protocols. Absorption of the magnesium stents made it difficult to quantify the concept of in-stent restenosis and amount of new tissue, reducing the options of making a by patient analysis of the vasomotion with relation to ‘neointimal’ growth. The small non-significant negative change in the BMS group is probably a function of the small group, however if one assumes no change in this group, the comparative non-parametric statistic still yields a significant change in the AMS group. In conclusion, this study is hypothesis generating, and suggests there may be the possibility of the return of more normal vascular biology including vaso-reactivity following stent resorption with bioabsorbable coronary stents which may have important implications for future stent design and the potential to result in beneficial long term outcomes.

