Abstract
Aims: Different distal protections devices have been developed to prevent embolisation during percutaneous coronary intervention (PCI) in atherosclerotic saphenous vein grafts (SVG). The purpose of this descriptive study was to characterise the composition of material captured by embolic protection device from saphenous vein graft interventions by light and electron microscopy, to determine the age of the thrombotic component and to relate the pathological findings to the clinical condition, lesion characteristics and to the use of conventional or membrane covered self-expanding stents during the intervention.
Methods and results: Forty consecutive patients treated with the FilterWire EX™ (Boston Scientific) during 42 SVG interventions were included. Plaque was found in 4.8%, thrombus in 16.7%, both plaque and thrombus in 69.0% of the filter bags, and 9.5% were empty. In 93% of the thrombus containing samples we found platelet aggregates that were formed within the last 10-15 minutes before fixation. This indicated platelet stimulation and aggregation during the PCI procedures. No relation was found between the composition of filter wire material and clinical condition, coronary lesion characteristics or the use of membrane covered self-expanding stents (Symbiot®).
Conclusion: The present study on material captured by embolic protein device after SVG interventions generated the hypothesis that these interventions were associated with acute platelet activation. In consequence, the use of glycoprotein IIb/IIIa inhibitors might be reconsidered in distal protected SVG interventions.
Introduction
Distal microembolism following percutaneous coronary interventions (PCI) in saphenous vein grafts (SVG) or atherothrombotic lesions in native coronary arteries may result in procedure related myocardial infarction and increased morbidity and mortality1-4. Therefore, different devices, such as aspiration catheters, filters and proximal or distal occlusion devices have been developed to reduce microembolism during the intervention5. In stenotic SVGs, the use of distal protection devices during stent treatment of these lesions has been associated with significant reduction of major adverse cardiac events6.
Histopathological studies using light microscopy on debris captured after SVG interventions have mainly revealed soft acellular atheromatous debris, typically found under the fibrous cap of atherosclerotic plaques7-9. In these studies thrombotic material was found as well, alone, or together with plaque elements.
In the present descriptive study, the purpose was to characterise the composition of filter wire material from saphenous vein graft interventions by light and electron microscopy, to determine the age of the thrombotic components and to relate the pathological findings to the clinical presentation, lesion characteristics and to the use of conventional or membrane covered self-expanding stents during the intervention10.
Materials and methods
Patients and procedures
A total of 40 patients were treated with the FilterWire EX™ (Boston Scientific) during SVG interventions from December, 2001 to November, 2003 at the Department of Cardiology, University Hospital of Northern Norway. Patient characteristics at baseline are shown in Table 1.

All patients received stents during the procedure. The stents were membrane covered self-expanding stents (Symbiot®, Boston Scientific), or conventional stents. Stent choice was at the discretion of the operator, and the operator should as far as possible avoid predilation prior to placing the filter wire distal to the lesion. Twenty-three of the patients suffered from an acute coronary syndrome. The procedural characteristics are given in Table 2.

Two patients had an intervention in two SVGs giving 42 samples for histopathological examination. Four of the samples prepared for light microscopy contained no material, leaving 38 samples for further examination. All patients were pre-treated with 500 mg of aspirin 12-24 h before the intervention and at the day of intervention. A clopidogrel loading dose (300 mg) was given 12-24 h before intervention followed by 75 mg daily. Heparin (80IU/kg) was given at the beginning of the procedure without routine control of the activated clotting time. None of the patients received glycoprotein IIb/IIIa (GP IIb/IIIa) inhibitors.
The FilterWire EX™ consisted of a filter bag with a pore size of 80 µm mounted on a PCI guide wire. The filter was collapsed and inserted into the SVG by a dedicated delivery catheter and placed distal to the index lesion. After removal of the delivery catheter, the filter bag expanded passively. The filter allowed passage of blood, but not embolic material to the distal vessels. At the end of the procedure the filter was removed by a retrieval catheter. Immediately after removal, 22 samples of the filter-bag were placed in a glutaraldehyde solution (McDowell’s fixative) for electron microscopic examination, 20 samples in a 10% buffered formalin for light microscopic examination. The weight and volume of the filter material was not assessed.
In six patients undergoing PCI for unstable angina a filter bag was placed counter current in the abdominal aorta for 5-10 minutes to serve as a control procedure.
Light microscopic examination
After fixation in 10% buffered formalin for approximately 24 hours the contents of the filter bags were removed by gently scraping and flushing with formalin, processed and embedded in paraffin. Tissue sections (5 µm) were cut and stained with haematoxylin and eosin (H&E), according to standard procedures.
The slides were evaluated using a light microscope (Olympus BX50), and examined for atherosclerotic material (foam cells, calcified deposits and necrotic material with or without cholesterol clefts) and thrombotic material (platelets and fibrin). The age of the thrombotic material was determined as described below.
Electron microscopic examination
The filter bags with contents were fixed in McDowell’s fixative for approximately 24 hours. The content of the filter bags was gently removed by scraping and then flushing with McDowell’s fixative before centrifugation. The precipitated material was processed and embedded in plastic (Epon-Araldite). The slides were cut in semi-thin sections (2 µm), stained with a 1% toluidine blue solution and examined by light microscopy to verify representative material, and to select areas for electron microscopical examination. Ultrathin sections of selected tissue areas were cut by the use of a diamond knife and later impregnated with heavy metal ions for contrast (uranyl acetate and lead citrate) before examination by a Hitachi (HU 12) transmission electron microscope with the primary magnification of 5000. The semi-thin sections were examined by a light microscope for the same parameters as the slides stained with H&E.
Evaluation of atherosclerotic material
Atherosclerotic material was identified with the finding of lipid macrophages and calcified deposits. Also, cholesterol clefts, collagen fibres and smooth muscle cells were considered as signs of atherosclerotic material.
Evaluation of the thrombotic material
To evaluate the time dependent changes in the thrombotic material we used the methods described by Jørgensen et al10-11, and Hovig et al12. They had shown that following stimulation with adenosine diphosphate (APD) or collagen, platelet aggregates were formed within 30 seconds. The aggregates were first loosely packed and had a granulated appearance in the light microscope. After 5 minutes aggregates were densely packed, and polymorphonuclear leukocytes started to appear on the surface. By about 10 minutes some polymorphonuclear leukocytes and fibrin were found on the surface of the aggregates. By 20 minutes there were more polymorphonuclear leukocytes and fibrin on the surface and between the surface-near platelets.
Transmission electron microscopy gives more detailed information than light microscopy on cellular organelle composition (ultrastructure). Platelets undergo a series of ultrastructural changes following stimulation by ADP as described by Jørgensen et al10-11. Already after 30 seconds loose aggregates of platelets could be identified. The platelets had changed from a disc shape to a rounded form with pseudopods and central organelles. Within 5 minutes they became more densely packed, like a jigsaw puzzle. After about 10 minutes fibrin strands and some polymorphonuclear leukocytes could be observed between and around clumped platelets at the periphery of the aggregates. By about 20 minutes the organelles were partly lost and vacuolisation and swelling of the pseudopods could be seen. At that time neutrophil polymorphonuclear leukocytes adhered to the surface in increasing amounts. In the following period of up to 5-6 hours there was a gradual transformation of the platelet mass to a fibrin-rich body, as fibrin threads were precipitated between the platelets, which gradually lost the grip on each other. We focused on the fresh thrombus material, and thrombus material more than 20 minutes old was not further characterised.
The material in our study was examined with both light microscopy and transmission electron microscopy and staged according to the criteria listed above.
The composition of the filter wire material was related to clinical condition (stable angina or acute coronary syndrome), age of grafts, TIMI flow, stent diameter, stent length, percent of the stenosis diameter by visual assessment and the use of membrane covered self-expanding stents (Symbiot®).
Results
Among the patients with stable angina pectoris there were only 2 patients with a significant release of myocardial biomarkers (> 3 times the upper limit of normal). 23 out of 40 patients had an acute coronary syndrome with myocardial biomarker elevation prior to intervention. This made it impossible to evaluate myocardial injury related to the procedure in these patients. TIMI flow before and after procedure, predilation, any visible thrombus present before stenting and angiographic evidence of distal embolisation are given in Table 2.
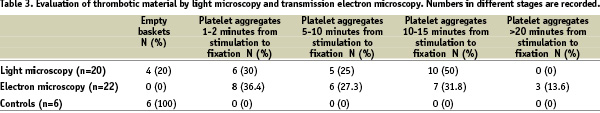
The results of the evaluation of the thrombotic material are given in Table 3.
Material from 38 samples was examined. In all samples containing material the embolic and the atherosclerotic components were identified and further investigation performed.
Of the 16 samples stained with haematoxylin and eosin for light microscopy, 13 (81.2%) had both atherosclerotic and thrombotic material, two (12.5%) only thrombotic material and one (6.3%) only atherosclerotic material.
In the 22 samples prepared for electron microscopy the material was first examined in toluidine blue stained sections by light microscopy to find representative areas of atherosclerotic and thrombotic material. Both atherosclerotic and thrombotic material was found in 16 samples (72.7%). Another 5 samples (22.7%) had only thrombotic material and one (4.5%) atherosclerotic material alone. Both the light microscopy examinations and the transmission electron microscopy examinations showed spatial proximity between the thrombotic and the atherosclerotic material, i.e. the thrombotic material appeared in most cases to adhere to atherosclerotic material.
The thrombotic material was examined and grouped into four categories according to the time dependent changes. In nine of the samples the thrombotic material was considered to be in different stages, some fresh and some older material in the same thrombus, and all the stages were noted (Table 3).
It was a common feature that the platelet aggregates on the surface were less altered than those located deeper in the deposits, indicating that platelets near the surface of the thrombus were activated more recently.
The material examined by light microscopy had loosely aggregated platelets together with erythrocytes in six samples, which correspond to freshly stimulated platelets (Figure 1A). Five of the samples had denser platelet aggregates without erythrocytes inside the aggregates and without fibrin deposits, corresponding to aggregates not older than ten minutes (Figure 1B). In ten cases there was, in addition, fibrin and polymorphonuclear leukocytes corresponding to platelet aggregates approximately 10-20 minutes old (Figure 1C).
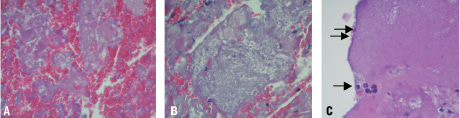
Figure 1. Embolic material from FilterWire stained with H&E and examined with light microscope. In A the platelets are loosely aggregated together with large amounts of erythocytes. In B the platelets have formed large aggregates. In C there are some polymorph nuclear leucocytes (single arrow), and a rim of fibrin (double arrows) is seen on the surface of the platelet aggregate.
The semi-thin slides stained with toluidine blue do not discriminate between the different structures as well as slides stained with H&E. Thus, no evaluation of the age of the thrombotic material was done on these slides.
The material examined by transmission electron microscopy had also thrombotic material of different ages within the same thrombus, and all were staged. Eight cases had loose platelet aggregates containing stimulated platelets with pseudopods that indicated stimulation less than 5 minutes prior to fixation (Figure 2A). In six cases there were denser platelet aggregates with pseudopods in close contact with each other, but without evidence of fibrin, indicating less than 10 minutes old aggregates (Figure 2B). Seven cases had small amounts of fibrin and some polymorphonuclear leukocytes in addition to the platelets being tightly packed together corresponding to fixation 10-20 minutes after stimulation (Figure 2C). Three cases had thick bundles of fibrin, degenerated swollen platelets with loss of intracellular granula and several polymorphonuclear leukocytes, features corresponding to thrombus material several hours old (Figure 2D).
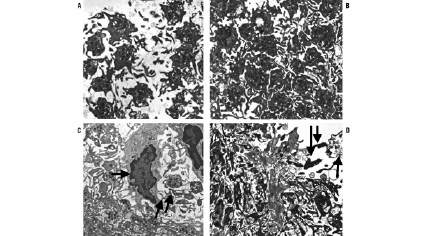
Figure 2. Embolic material from FilterWire EX™ examined by transmission electron microscopy. In A loosely aggregated platelets with pseudopods are seen. In B the platelets are aggregated more densely like a jigsaw puzzle. In C there are polymorphonuclear leukocytes on the surface of a thrombus (single arrow) and small amounts of fibrin (double arrows). In D the platelets are disaggregated with swollen pseudopods (single arrow) and there are large amounts of fibrin (double arrows).
The filter bags from the six controls contained a small amount of visible erythrocytes, but no thrombotic or atherosclerotic material was identified when evaluated with light microscopy. Electron microscopy showed a group of approximately ten freshly stimulated platelets in one of the filter bags. These platelets were stimulated within the last minute before being fixated. The amount of platelets in this filter bag was much less then the amount found after SVG PCI, and was not considered to be a thrombotic aggregate (Table 3).
No relation was found between the composition of the filter wire material and the clinical condition (stable angina or acute coronary syndrome), age of grafts, TIMI flow, stent diameter, stent length, and percent of stenosis diameter by visual assessment and the use of membrane covered self-expanding stents (Symbiot®).
Discussion
The present descriptive study demonstrated embolic material composed of freshly activated platelets captured in the distal protection filter during PCI of degenerated saphenous vein grafts. The platelet activation had occurred within 20 minutes prior to fixation. Thus, the platelets had been activated during the PCI procedure despite treatment with aspirin, heparin and clopidogrel. In several of the samples we found the platelet aggregates on the surface less altered than those deeper in the deposits, indicating that they were activated more recently. A similar stratification has been described in other studies and is regarded characteristic of mural growing thrombi12.
The use of distal protection device during stenting of stenotic venous grafts is associated with a significant reduction of major adverse events compared with conventional stenting6. We found no difference regarding presence of captured material, or composition of captured material when using membrane coated self-expanding stents or conventional stents. This correlates well with a recent study that showed that membrane covered self-expanding stents did not prevent distal embolisation in SVG PCI13.
It is widely recognised that intravascular platelet activation is associated with acute coronary syndromes. However, the frequency and clinical importance of microembolisation during acute coronary syndrome has only recently been appreciated3, although early studies with ADP-induced transient platelet aggregation in the microcirculation of the myocardium have shown profound circulatory effects with permanent ischaemic damage to the myocardial tissue in animals10. Post-mortem studies of patients, who died after thrombus mediated heart attack, have revealed platelet microembolism and, more rarely, microembolism of atherosclerotic debris in small intramyocardial arteries in a high proportion of cases3,14-17. The deposition of microembolic material to the microcirculation may cause a cascade activation of thrombosis and inflammation leading to myocardial damage18.
Atherogenesis is accelerated in saphenous vein grafts, and severe atherothrombosis may develop within a few years after coronary artery bypass grafting3,4,19. The atherothrombotic lesions in vein grafts are generally larger and contain more lipid, foam cells and thrombus, but less calcification than atherothrombotic lesions in native coronary arteries. Endothelial defects, fissures and small haemorrhages are common in the atherosclerotic intima. Endothelial injury may also take place during a saphenous vein graft PCI, and even a small endothelial injury may activate platelets20.
To test the possibility that the filter bags per se could stimulate the formation of platelet aggregates, six filter bags were placed counter current in the abdominal aorta in patients undergoing PCI. Only one filter bag contained a minimal amount of captured platelets, which had been stimulated within the last minutes before fixation. Thus, it is unlikely that the filter per se stimulates significant platelet aggregation, and is responsible for the histological changes we have found in the filter material captured during SVG treatment. This is also supported by the fact that four of the filter bags from the interventions were empty.
Troponin elevations in non-ST-elevation myocardial infarction indicate small infarctions of thrombotic, but not necessarily microembolic, origin. It may be a result of reduced flow to subendocardial regions because of a critical stenosis, but may also be a result of distal microembolism. Our demonstration of acute platelet microembolism during saphenous vein graft interventions suggests that these patients might benefit from intensive platelet inhibition and further encourages the use of distal protection devices during these procedures.
Glycoprotein IIb/IIIa receptor inhibition reduces the incidence of ischaemic complications secondary to prevention of platelet aggregation, thrombus formation and distal embolisation during interventions in native coronary arteries, but has not been shown to reduce complications resulting from distal embolisation in SVG interventions4,21. This might be due to the mixed composition of the SVG debris, as found in the filters of the present study, and not necessarily to lack of effect on platelet inhibition. As underlined by Roffi et al4, the studies in their meta-analysis were performed without use of distal protection. Therefore, the distal embolisation might have been caused by atherosclerotic material in the GPIIb/IIIa treated patients.
In conclusion, the present study on filter wire material from SVG interventions generated the hypothesis that these interventions are associated with acute platelet activation. In consequence, the use of GP IIb/IIIa inhibitors may be reconsidered in distal protected SVG interventions.
Acknowledgements
We thank Kate Myreng for valuable technical work.