Abstract
Stent thrombosis is one of the major concerns after drug-eluting stent implantation. Multiple mechanical causes (i.e. stent under-expansion, edge dissection, geographic miss, residual stenosis, incomplete stent apposition and aneurysm) have been postulated. These features are easily identifiable by intravascular ultrasound. However, it is uncertain which of them are inextricably related to stent thrombosis, primarily due to the low number of such patients studied by IVUS in case control studies.
Complementary to greyscale IVUS, tissue characterisation by IVUS radiofrequency data (RFD) analysis has the potential to add valuable information on the pathogenesis of stent thrombosis by providing information on plaque composition, specifically on the amount of necrotic core and its location (superficial or deep). However, the clinical utility of IVUS-RFD analysis in this context has yet to be demonstrated.
Introduction
In current practice, one of the most important concerns for the interventionalist is stent thrombosis, primarily of drug-eluting stents (DES)1-5. Among the multiple postulated causes, there is a large group encompassed in so-called mechanical causes (i.e. stent under-expansion, edge dissection, geographic miss, residual stenosis, incomplete stent apposition [ISA] and aneurism [extreme ISA]). These were also formerly related to thrombosis of bare metal stents (BMS). In fact, for this stent type, it has been reported that in 78% of the patients with stent thrombosis at least one mechanical cause is present using greyscale intravascular ultrasound (IVUS)6. Furthermore, ISA at long-term follow-up seems to be more frequent after sirolimus-eluting stent (SES) implantation than after BMS implantation7. Extreme positive remodelling after DES implantation is one of the main processes involved in cases with late acquired ISA. More importantly, ISA has recently been linked to thrombotic coronary events4,8.
Complementary to greyscale IVUS, tissue characterisation by IVUS radiofrequency data (RFD) analysis has the potential to add valuable information on the pathogenesis of stent thrombosis by providing information on plaque composition, specifically on the amount of necrotic core (NC) and its location (superficial or deep). Although IVUS-RFD analysis is a new technique and there is a long way ahead to prove its clinical value in the context of stent thrombosis, in this report we describe pathological findings that have been related to stent thrombosis that are identifiable by IVUS-RFD.
We also describe the current evidence that relates greyscale IVUS findings with stent thrombosis.
Definitions of the mechanical causes of stent thrombosis (Figure 1)
Stent under-expansion index, defined as minimum stent cross-sectional area (CSA) ÷ mean of proximal and distal reference areas4,8.
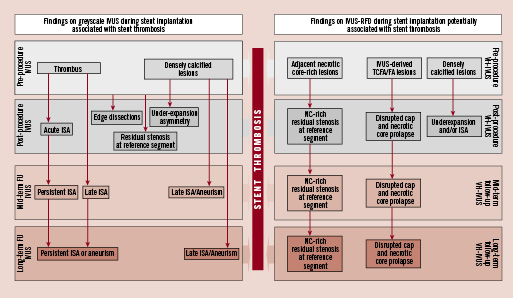
Figure 1. This illustration displays the potential use of greyscale IVUS and IVUS-radiofrequency data (RFD) analysis across the different stages of the interventional procedure and imaging follow-up of the patients. Greyscale IVUS can categorise plaque types and the acute result of the intervention, as well as provide useful information on some characteristics that have been related to stent thrombosis such as incomplete stent apposition (ISA), edge dissection, residual stenosis, under-expansion and thrombus. IVUS-RFD could provide valuable information on the intact plaque, especially on necrotic core (NC) amount and location.
Significant residual reference segment stenosis, defined as the combination of a reference segment minimum lumen CSA <4 mm2 plus a plaque burden >70%9.
Geographical miss, defined as a mismatch of intended lesion and balloon-injured targets with subsequent stent deployment sites10. Unlike previous definition, geographical miss also refers to balloon-injured areas left untreated.
Incomplete stent apposition, defined as the lack of contact of at least one stent strut with the vessel wall, not encompassing a side branch. This can be detected immediately after stent implantation (acute or post-stenting ISA) or at follow-up (six months and beyond - late acquired ISA). (Figure 2).
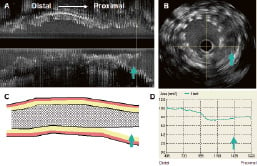
Figure 2. Panel A shows a longitudinal view of an IVUS pullback. The arrow points to a proximal segment that is not apposed to the vessel wall. Panel B is the corresponding cross-sectional area (CSA). The arrow shows multiple stent struts that are not apposed. Panel C is a diagrammatic representation of the longitudinal view. Notice the lack of stent apposition signalled by the arrow. Panel D indicates the stent CSA throughout the segment. An important decrease in stent area is seen at the proximal part.
Edge dissection, defined as the presence of a flap in close proximity to stent edges.
Aneurysm, defined as an enlargement of both the external elastic membrane (EEM) and lumen area >50% of the proximal reference segment11.
Greyscale IVUS
Incomplete stent apposition has been postulated to be one of the potential causes of stent thrombosis. Yet, is ISA truly highly prevalent in patients with stent thrombosis after DES implantation? Or is stent thrombosis a rare event among patients with ISA? This is not clear, the reported prevalence of ISA post-stenting among DES studies (sirolimus, paclitaxel ([PES]) and everolimus-eluting stents [EES]) varies from 2.4 to 34.4%12-18. On the other hand, although late-acquired ISA has, by definition, the same IVUS appearance as post-stenting ISA, its postulated mechanisms are completely different including progressive expansion of the vessel wall, thrombus resolution and lack of tissue growth around the stent struts17. In the literature, the incidence ranges from 0.0 to 16.7%12-14,16-21. Possible explanatory reasons for the wide spread of occurrence across all reported stent studies are the following: i) lesion-related (calcified, eccentric, chronic total occlusions or thrombus)17; ii) procedure-related (absence of postdilatation and IVUS-guidance); iii) device-related (DES > BMS); and iv) corelab-related (inter-analyst and inter-corelab variability). Of note, no clinical events related to ISA have been reported in these trials.
The STLLR trial documented that geographical miss occurred in 66.5% of implantations and was an additional risk for late acute coronary events following drug-eluting stent implantation10. Thus, this suggests that angiographic-guided implantation of DES may be not satisfactory. In this regard, IVUS easily identifies incomplete lesion coverage, stent under expansion and ISA, yet remains infrequently used (approximately 7% of cases in the United States)22. Although IVUS-guided stenting could potentially improve stent implantation, the cost-effectiveness of routine IVUS use in this context has yet to be evaluated. More importantly, whether this approach would impact the incidence of late stent-related clinical events is unknown. However, we do not think all cases are suitable for IVUS-guided stenting. In this era of more liberal DES use, interventionalists frequently have to treat vessels with complex anatomy (e.g. severe tortuosity), in which even with IVUS-guided stenting, complete apposition may not be fully ensured. Thus, a careful analysis of the coronary anatomy prior to stent implantation may help operators to select cases in which IVUS imaging is safe and the information useful.
Clinical studies of patients with stent thrombosis and intravascular ultrasound imaging
Fujii et al9 compared 15 patients treated with SES who experienced early stent thrombosis with 45 patients who had no evidence of SES thrombosis. Incomplete stent apposition was found in 13% of patients with SES thrombosis vs. 16% of controls. The minimum stent CSA was 4.3±1.6 mm2 in patients with SES thrombosis compared with 6.2±1.9 mm2 in controls (p <0.001); the stent under-expansion index was smaller in SES thrombosis (0.65±0.18 vs. 0.85±0.14; p < 0.001), and the residual reference segment stenosis was 67% in the SES thrombosis group vs. 9% in the controls, p <0.001.
In another case control study of 13 patients (14 DES with early thrombosis)23, the minimum stent CSA was also smaller as compared to controls (4.6±1.1 vs. 5.6±1.7 mm2; p <0.05), with 11 of 14 stents (79%) having a minimum stent CSAs < 5.0 mm2 compared with 12 of 30 (40%) in the control group (p=0.04). These authors also found larger residual reference segment stenosis in patients with thrombosis.
Mintz et al21 reported the comparison of 15 cases of DES with early thrombosis with 45 cases of DES restenosis. In line with the above-mentioned reports, the minimum stent CSA was smaller in DES thrombosis lesions (3.7±0.8 vs. 4.9±1.8 mm2; p=0.01). Independent predictors of stent thrombosis were diffuse stent under-expansion (odds ratio [OR], 1.5; p=0.03) and proximal location of the site of minimum stent CSA (OR, 12.7; p=0.04).
Siquiera et al24 reported late-acquired incomplete stent apposition in 10 out of 195 patients (seven with SES, three with PES) studied with IVUS at six months; two out of these 10 patients with late-acquired ISA had stent thrombosis at 331 (PES) and 1,152 days (SES).
A report of two cases with DES thrombosis (one SES and one PES) revealed extensive positive remodelling (increase in vessel volume of 19.7 and 38.6%) leading to large areas of late-acquired incomplete stent apposition in both cases by means of serial angiography and IVUS25.
More recently, Cook et al8 reported 11 patients with very late DES thromboses and compared them with 198 controls that had undergone routine IVUS follow-up but did not develop stent thrombosis. Incomplete stent apposition was present at the time of thrombosis in 77% of patients with very late DES thromboses vs. 12% of controls imaged during routine follow-up; p <0.0001. The authors suggested that incomplete stent apposition may play a role in the pathogenesis of this adverse event.
The latest report available in the literature is by Alfonso et al4 who reported 26 patients with DES thrombosis out of 1,974 patients treated with DES during the same period, resulting in a two-year stent thrombosis incidence of 1.3%. Only 12 were included in the IVUS substudy. Thrombotic occlusion was seen in all patients by IVUS. Severe stent under-expansion and significant residual reference segment stenosis were again the common denominators in this series. Incomplete stent apposition was detected in 50% of the patients (three subacute, three late thrombosis), and major side branches jailed by the stent were seen in 67% of the patients. According to the Multicentre Ultrasound Stenting in Coronaries Study (MUSIC) criteria26, deployment of the stents was suboptimal in all patients with stent thrombosis. In contrast with other studies, the minimum stent area was not small (9±3 mm2). After re-intervention, residual thrombus was present in all patients (17±7% of stent volume post-intervention vs. 51±22% pre-intervention, p= 0.001).
We can therefore conclude that incomplete stent apposition is highly prevalent among patients with DES thrombosis that have been studied by IVUS. In addition, stent under-expansion and residual reference segment stenosis were also constantly associated with DES thrombosis.
Table 1 summarises the IVUS findings that have been reported among patients with stent thrombosis.
IVUS Radiofrequency data analysis
Our knowledge of the pathophysiology of late DES thrombosis is derived from pathologic samples27-30. It has been demonstrated that DES cause substantial delayed healing (the most common cause of late DES thrombosis at autopsy) characterised by the lack of complete re-endothelialisation and persistence of fibrin when compared with BMS31. This cannot be assessed by any IVUS modality due to its limited axial resolution (100 µm).
Other proposed pathological mechanisms of coronary stent thrombosis are stenting of necrotic core-rich plaques with extensive tissue prolapse and plaque disruption in the proximity of the stented arterial segment27,31. (Figure 3). IVUS-RFD is able to characterise necrotic core with high sensitivity and specificity32. This technique also provides geometrical analysis of each frame, allowing us to have the combined assessment of necrotic core and plaque size; indeed this relationship has been tested in 25 patients with acute coronary syndromes in whom an increment in plaque size was followed by an increase in the NC33. Thus, pre-stenting imaging using IVUS-RFD can give us an insight not only into the extent of plaque but also on the extent of necrotic core within and beyond the intended stenting segment. This latter assessment is important since stenting has been lately performed, under conventional angiography guidance, from “normal to normal” arterial segments; however, disruption of adjacent necrotic core-rich areas that are angiographically disease-free could be avoided by IVUS-RFD pre-stenting investigation. In this context, we studied 24 patients in whom 26 stented segments (by angiography stenting was performed from “normal to normal” coronary segments) using IVUS-RFD were assessed. We observed that necrotic core rich areas were left unstented (5mm-proximal edge mean necrotic core 17.0±13.5% and 5 mm-distal edge 18.5±16.5%); however, no stent thrombosis has been observed in this small cohort of patients at six months follow-up. It has yet to be determined in a larger population whether incomplete coverage of necrotic core-rich coronary plaques or disruption of adjacent necrotic core areas by DES impact on long-term clinical events.
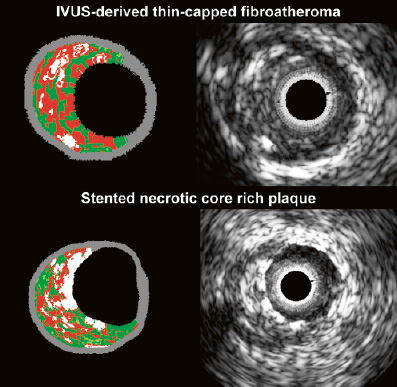
Figure 3. Greyscale IVUS frame and its corresponding IVUS-radiofrequency data frames of an IVUS derived thin cap fibro-atheroma (two upper frames). At the bottom, a stented necrotic core-rich plaque is shown. Notice the presence of stent struts at the lumen in the color picture as “dense calcium” on top of a confluent area of necrotic core. Virtual histology color code: green is fibrous, greenish is fibro-fatty, red is necrotic core and white is dense calcium.
Bifurcation lesions are the preferable location of TCFAs; in clinical studies this subset of lesions has been related to stent thrombosis4; we therefore hypothesised that the evaluation of the location and amount of the necrotic core is crucial. Thus, not only may the bifurcation stenting technique change accordingly, mainly by avoiding overlapping segments on these NC-rich areas, but also the stent used to treat these lesions. The desirable objective is a device that offers: a) mechanical stabilisation, b) promotion of vascular healing and c) reduction of inflammation. Nevertheless, it is worth mentioning that although related to a different physiopathological process and other arterial segment – restenosis following carotid stenting – the dissection of a lipid-rich, inflammatory plaque has been recently associated with reduced risk of restenosis.34
Theoretically, IVUS-RFD enables us to characterise in vivo TCFA - IVUS-derived thin-capped fibro-atheroma (IDTCFA); these lesions may pose a distinctive high risk of stent thrombosis, due to the fact that they contain large amount of necrotic core located superficially. We have recently developed software to quantify the amount of necrotic core in contact with the lumen, enabling refinement of our analysis. Our current definition of an IDTCFA is a lesion fulfilling the following criteria in at least three CSAs: 1) plaque burden > 40%; 2) confluent necrotic core >10% in direct contact with the lumen (i.e., no visible overlying tissue) in the investigated CSA; all consecutive CSAs having the same morphologic characteristics are considered as part of the same IDTCFA lesion35. In a recent study, using this refined definition of TCFA as assessed by IVUS-RFD, in patients with ACS who underwent IVUS imaging of all three epicardial coronaries, on average, there were two IDTCFAs per patient, half of which showed outward remodelling35 (Figure 3).
Post-stenting analysis by IVUS radiofrequency data analysis is limited since this technique lacks proper validation in this respect. An approach to get around this limitation could be to perform pre-stenting and post-stenting IVUS-RFD to assess the acute changes in terms of composition and relate this to the follow-up findings. Thus an IVUS-RFD clinical study that investigates the relationship between plaque composition of the intended stent segment (including its 10 mm proximal and distal segments) and development of stent thrombosis is still to be scheduled.
Conclusions
Although there are several potential mechanical causes of stent thrombosis that can be detected during the index procedure by greyscale IVUS as well as by repeated imaging at follow-up, it is uncertain which of them are inarguably related to stent thrombosis, primarily due to the low number of patients with stent thrombosis studied by IVUS in case control studies where causality is difficult to assess. A longitudinal and prospective study, adequately powered to demonstrate the mechanical factors potentially identifiable by IVUS and related to stent thrombosis is still awaited.

