Abstract
Aim: To compare clinical efficacy and safety of stents eluting limus drugs from biodegradable polymer – Nobori, or durable polymer – Cypher.
Methods and results: From May to August 2006, 107 patients with 142 coronary artery lesions were treated with either Nobori, Biolimus A9 eluting stent (54) or Cypher, Sirolimus eluting stent (53) in five centres. The two groups were well matched for baseline clinical and angiographic characteristics. The in-stent late loss at nine months, the primary endpoint of the study, was 0.10±0.26 mm in Nobori, and 0.13±0.44 mm in Cypher arm (p=0.660) confirming the hypothesis of the similarity between the two stents. In-stent diameter stenosis of 13±10% with Nobori was significantly lower than 20±12% with Cypher stent (p=0.002) without significant difference in binary restenosis (1.7% in Nobori and 6.3% in Cypher arm; p=0.32). The rate of major adverse cardiac events at 12 months was 1.9% with Nobori and 4.1% with Cypher stent.
Conclusions: The nine months angiographic data from NOBORI CORE study demonstrate that Biolimus A9 has similar anti-proliferative efficacy to Sirolimus as judged by in-stent late loss and restenosis rate. Low frequency of adverse cardiac events at 12 months indicates that both stents are safe and effective in the studied population.
Introduction
A large number of clinical trials have demonstrated the clear superiority of drug eluting stents (DES) over bare metal stents for the prevention of restenosis and the need for repeat revascularisation.1-5 Because of their efficacy in limiting neointimal hyperplasia after percutaneous coronary interventions, drug eluting stents have become routine therapy in clinical practice and set forth comparative standards for the evaluation of novel stent technologies. Currently available DESs employ durable polymers of various formulations and different types of anti-proliferative agents, mainly cytostatic drugs from the limus family or cytotoxic drugs from the taxane group. Due to the suspected association of the first generation DESs with the late adverse clinical outcomes a clear need for the further developments has been identified.6-8 From the clinical perspective any new drug eluting stent is expected to retain similar anti-restenotic efficacy, but should have better safety profile.
The Nobori drug eluting stent represents a novel concept in which an anti-proliferative drug Biolimus A9™ is eluted from a biodegradable polymer. Specific design of stent coating, the efficacy of the drug, and the degradation of the polymer over time are expected to both, reduce neointimal hyperplasia and ensure long term safety. The Nobori stent has been studied earlier in randomised trials versus Taxus Express or Taxus Liberté, both eluting paclitaxel.9,10 The NOBORI CORE is the first comparative study of the Nobori stent employing biodegradable polymer versus another limus drug eluting stent with durable polymer. This is a multicentre, prospective, similarity trial with a consecutive enrolment of the patients.
Methods
Patient population
Patients older than 18 years, with ischaemic heart disease due to lesions in native coronary arteries were considered for enrolment. Angiographic inclusion criteria were a reference vessel diameter of 2.5 mm to 3.5 mm and a lesion length of > 5 mm. Major clinical exclusion criteria were intolerance to aspirin, heparin, clopidogrel bisulfate, ticlopidine, drugs similar to Biolimus A9 (Sirolimus, Tacrolimus, Everolimus, Zotarolimus), contrast media, and stainless steel; platelet count <100,000 >700,000 cells/mm3 or a WBC <3,000 cells/mm3; myocardial infarction within 48 h preceding the index procedure. Main angiographic exclusion criteria were left main disease, severe calcification, evidence of thrombus or severe tortuosity.
The study patients were enrolled in five medical institutions (listed in Appendix 1). The study was conducted according to the Declaration of Helsinki, ISO 14155, and respecting all country specific regulatory requirements. The protocol was reviewed and approved by the competent authority and all relevant ethics committees and written informed consent was obtained from all patients. This was not a randomised study but all eligible consecutive patients in each institution were treated with Nobori stent in a first, and with Cypher stent in a next clearly predefined enrolment period, or vice versa.
The Nobori stent
The Nobori drug eluting stent system (Terumo Corporation, Tokyo, Japan) consists of three components: the stainless steel S-stent and its delivery catheter, a drug carrier, poly-lactic acid (PLA), and an anti-proliferative substance, Biolimus A9™ (Biosensors International). The biodegradable polymer, PLA, has been used in a variety of medical applications and is completely metabolised in mammalian systems to carbon dioxide and water via conversion to pyruvic acid and entrance into the Krebs cycle. The Nobori DES is coated only abluminally with the matrix containing Biolimus A9™ and PLA (15.6 µg each per 1 millimetre of stent length). The drug-polymer matrix is designed to release the drug simultaneously with the polymer degradation in a process lasting between nine and 12 months. The coating design, along with the lipophilicity of the drug, is expected to optimise drug distribution and to reduce its release into the peripheral circulation.
Biolimus A9™ is an analogue of Sirolimus (rapamycin), possessing immunosuppressive and anti-proliferative activities. The potency of locally delivered Biolimus A9™ to prevent neointima proliferation has first been studied in the STEALTH trial11 and confirmed in NOBORI 1 clinical trial. 9,10
Coronary stent procedure
Stents were implanted according to the standard clinical practice with a mandatory predilatation. At least 100 mg of aspirin was administered daily to all patients before the procedure and indefinitely thereafter. A loading dose of Clopidogrel was recommended pre- or peri-procedurally and subsequently at 75 mg/day for six months, to all patients receiving stents. The use of glycoprotein IIb/IIIa inhibitors was at the physician’s discretion.
Nobori™ stents were available in lengths of 8, 14, 18, 24 and 28 mm and in diameters of 3.0 and 3.5 mm. Cypher® stents (Cordis Corporation) were available in lengths of 8, 12, 16, 20, 24, 28 and 33 mm and in diameters of 2.5, 3.0 and 3.5 mm.
Patients follow-up
All patients were scheduled to have a repeat angiography at nine months ±30 days and clinical follow-ups at 30 days, nine and 12 months and annually up to five years. The clinical follow-up included assessment of angina status, monitoring of cardio-active and anti-thrombotic drug use, interim hospitalisations, occurrence of major adverse cardiac events, and any invasive and non-invasive diagnostic test or interventional treatment that has occurred since the previous contact. In this study there were 10 patients from Montenegro, the republic which became an independent country by the time of follow-up. Therefore these patients had neither control angiography nor hospital visits at nine and 12 months. Seven patients were contacted by telephone, while three patients worked on long distance ships and could not be contacted before database was closed. Those patients still are part of the study and will be contacted for further follow-ups.
Endpoints and definitions
The primary endpoint was angiographic in-stent late-loss at nine months post-procedure defined as the difference between the post-procedure minimal lumen diameter (MLD) and the follow-up angiography MLD. Secondary endpoints were MACE, defined as the composite of cardiac death, myocardial infarction, emergent cardiac bypass surgery, and target vessel revascularisation (TVR) at 30 days, nine, and 12 months, and annually up to five years; angiographic in-stent and in-segment binary restenosis rate (defined as > 50% diameter stenosis); lesion success, defined as the attainment of < 30% residual stenosis by visual assessment and/or < 50% by QCA using any percutaneous method; device success defined as achievement of a final diameter stenosis of < 50% by QCA, and/or <30% by visual assessment, using the assigned device only; procedure success defined as achievement of a final diameter stenosis of < 30% by visual assessment and/or < 50% by QCA, using any percutaneous method, without the occurrence of death, Q wave or non-Q wave MI, or repeat revascularisation of the target lesion during the hospital stay. MI was defined either as the development of pathological Q-waves in at least two contiguous leads with or without elevated cardiac enzymes or, in the absence of pathological Q-waves, as an elevation in creatinine kinase levels to greater than twice the upper limit of normal in the presence of an elevated level of CK-MB fraction and at least one of the following: symptoms or ECG changes indicative of ischaemia or coronary artery intervention.
Target lesion revascularisation (TLR) was defined as any clinically-driven repeat percutaneous intervention of the stented lesion including 5 mm proximal and distal from the edge of the stent, or bypass surgery of the target vessel that was performed for a clinical indication and was due to restenosis or closure of the target lesion. Target vessel revascularisation was defined as clinically driven revascularisation of any segment of the index coronary artery, which was in physical contact with any component (guiding catheter, guide wire, balloon catheter, etc.) of the angioplasty hardware during the initial procedure. TLR or TVR were considered clinically driven if prompted by a positive functional study, by ischaemic ECG changes at rest in a distribution consistent with the target vessel, or by ischaemic symptoms with an in-lesion diameter stenosis > 50% by QCA or if lesion diameter stenosis was more than 70%. Stent thrombosis was defined as confirmed thrombus within the stented vessel at the time of the clinically driven revascularisation for documented ischaemia (chest pain and ECG changes), representing abrupt or sub-abrupt closure. Any unexplained death or any Q-wave myocardial infarction in the territory of the stented segment within the first 30 days, were considered a surrogate for stent thrombosis when angiography was not available. It was classified as acute if it occurred within the first 24 hours, sub-acute up to 30 days, and late after 30 days. Late thrombosis was defined as MI attributable to the target vessel with angiographic documentation of thrombus or total occlusion at the target site more than 30 days after the index procedure in the absence of an interim target vessel revascularisation.
An independent clinical event committee reviewed and adjudicated all major adverse cardiac events. The committee was composed of interventional cardiologists not associated with the study.
Quantitative coronary angiography evaluation
The independent reviewers analysed all pre-, peri- and post-procedural angiographic images using Medis (Medical Imaging System, Leiden, The Netherlands) software. In each patient, the stented segment and the peri-stent segments (defined as the length of 5 mm proximal and distal to the stent edges) were analysed. The following parameters were computed: reference vessel diameter (RVD), minimal lumen diameter (MLD) and percent diameter stenosis. Binary restenosis (BR) was defined as diameter stenosis of a more than 50% at follow-up. Late loss (LL) was defined as the difference between MLD post-procedure and MLD at follow-up.
Statistical analysis
This study was designed to show the similarity between Cypher Sirolimus eluting stent and Nobori Biolimus A9™ eluting stent on the primary endpoint of in-stent late loss at nine months. A 0.20 mm difference in late loss is considered to be the minimum clinically important difference.
From the late loss of Cypher stent in the SIRIUS trial (0.18 mm ±0.44 mm, mean±SD) and the results of other drug eluting stents and bare metal stents available at that moment, it was considered that the upper confidence limit should be between 0.40 mm and 0.50 mm. On the basis of the result from the STEALTH study, the late loss of Nobori stent at nine months was extrapolated to 0.25±0.50 mm.11
Taking these estimations into account, a 95% confidential interval was set at 0.15 mm.
Based on the assumption above, a sample size of 46 patients was estimated for each group of the study using the following formula for confidence intervals on means12 where “n” is a sample size, “SD” is a standard deviation, “Width” is the width of a desired 95% confidence interval.
Taking into account multiple vessels treatment and the attrition rate, the total sample size was increased to 102 patients (51 patients in each group).
The primary endpoint and all trial endpoints were analysed on the intent-to-treat population. Clinical events including death, MI, and revascularisation are reported on a per patient basis. For patients with multiple lesions, a failure of any lesion was counted toward the composite event rate. For continuous variables, differences between the treatment groups were examined by analysis of variance, while Fisher’s exact test was used for categorical variables. To assess the impact of vessel size on the late loss, a separate analysis of the lesions subset in the vessels smaller than 3.0 mm was also performed. All statistical analyses were performed using SAS statistical software, version 9.1 (SAS Institute Inc, Carry, NC, USA).
Results
Baseline patients and procedure characteristics
From May to August 2006, 107 patients were treated with either the Nobori Biolimus A9 eluting stent (54 patients) or the Cypher Sirolimus eluting stent (53 patients). The two groups were well matched with respect to baseline clinical variables (Table 1).
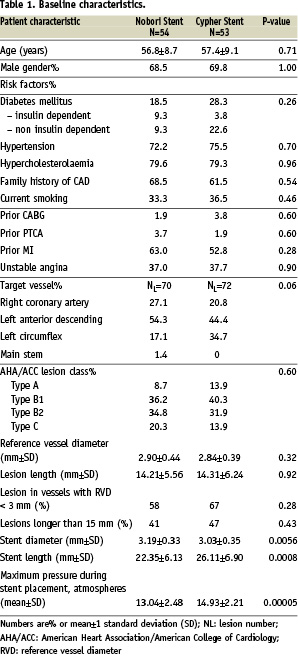
The Figure 1 shows the enrolment of patients and the compliance with follow-up.
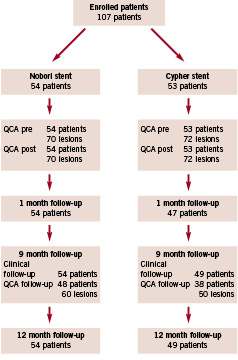
Figure 1. Patient flow.
The lesions in the two arms were treated similarly by conventional technique. Per patient 1.30 lesions were treated in Nobori and 1.36 in Cypher arm.
Both stents had excellent (100%), lesion, device and procedure success rates. In Nobori arm 55% of lesions were complex (B2 and C) compared to 46% in Cypher arm.
Quantitative coronary angiography findings
Angiography at nine months was completed in 86 (80%) patients. There were no significant differences in any pre-procedural angiographic lesion characteristics, however significantly higher acute gain in the Nobori arm resulted in statistically significant difference in post-procedure in-stent MLD and RVD (Table 2).
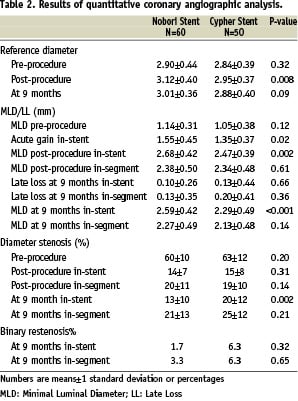
Mean in-stent late loss at nine months, the primary endpoint of the study, was similar in both groups with 0.10±0.26 mm in Nobori and 0.13±0.44 mm in Cypher stent (p=0.660), confirming the primary hypothesis of the similarity between the two stents. No significant differences in angiographic restenosis rate between the two groups were noted. To assess whether the difference in RVD detected post-procedure could have an impact on the primary endpoint we calculated the late loss of the lesions subset in the vessels smaller than 3 mm. For this subset the in-stent late loss was 0.12±0.27 mm and 0.14±0.40 mm (p=0.88) for Nobori and Cypher stent respectively (data not shown).
Clinical outcomes
There were no adverse cardiac events recorded during the index hospitalisation in any of the groups.
Clinical follow-up at 30 days was completed in 97% and at nine and 12 months in 96% of patients. At one month follow-up, one subacute stent thrombosis occurred in Nobori arm. The patient discontinued dual antiplatelet therapy one week after the procedure and two days later was admitted to hospital with the signs of myocardial infarction. On the baseline procedure the patient presented with unstable angina having two lesions in proximal and distal segment of right coronary artery (RCA). Both lesions were treated with the implantation of Nobori stents. Angiogram on re-admission showed a thrombus in the middle segment (previously untreated) of RCA. The patient was successfully treated with the implantation of a Taxus stent in the middle segment of RCA without overlapping any of the Nobori stents. The angiography after the implantation of the Taxus stent showed satisfactory distal flow indicating that the Nobori stent implanted in a distal segment was not occluded.
There were no further major adverse cardiac events reported between one and nine months.
In the Cypher arm two patients underwent repeat percutaneous intervention subsequent to nine months angiographic follow-up driven by in-stent restenosis and positive functional ischaemia studies.
At nine months (300 days) follow-up the MACE rate was 1.9% in Nobori arm and 4.1% in the Cypher arm and it did not change up to 12 months (380 days).
At one year follow-up 90.6% of patients in Nobori and 83.7% of patients in Cypher arm did not have any symptoms of angina.
Compliance with dual antiplatelet therapy
Dual antiplatelet therapy per protocol was recommended for six months however, 95% of patients were still taking both, aspirin and clopidogrel or ticlopidine at 12 months.
Discussion
The NOBORI CORE clinical trial confirmed similarity of the Nobori Biolimus A9™ eluting stent to the Cypher, Sirolimus eluting stent, with respect to late loss at nine months. Both stents showed excellent safety profile up to 12 months follow-up. Taking the limited exclusion criteria, the consecutive enrolment of eligible patients, high frequency of unstable angina and multiple vessel disease, and considerable number of treated long lesions and smaller vessels, we believe that the population in this study could be considered as similar to daily practice and representative of accepted indications for DES use. In our country DES is not the first choice for the primary PCI, therefore we did not treat patients in the course of acute myocardial infarction.
The type and the complexity of the lesions were similar in both arms, except for larger post-procedure in-stent MLD and RVD in Nobori arm. This difference is a result of a slightly smaller pre-treatment MLD in Cypher arm combined with a significantly higher acute gain which was achieved by Nobori stent, despite the significantly lower deployment pressure. The difference in post-procedure MLD could have impacted the restenosis rate and late loss as previously described in the literature.13,14 To test this hypothesis we performed a separate analysis of angiographic results in small vessels and found very similar low late loss in both stents confirming their efficacy in this lesion subset. Although lower compliance with angiographic follow-up in Cypher arm could have an influence on the primary endpoint we did not observe any inconsistency between our results, and findings of the other studies which assessed late loss with Cypher stent.1,2,15
The impact of oculo-stenotic reflex at angiographic follow-up was not so apparent.16,17 Investigators were requested to send a commitment sheet related with the intention to perform revascularisation before angiographic follow-up. This might have reduced revascularisations in the absence of clinical symptoms.
This study was not powered to assess similarity of the two stents in any of the clinical endpoints but a very low frequency of major adverse cardiac events in both cohorts of the patients is worth noting.
The overall results of NOBORI CORE study are in accordance with literature data available for both Cypher,1,2,15 and Nobori stents.9,10
The results of NOBORI CORE study add further evidence to the safety and efficacy of Nobori stent, and confirm that both limus drugs, Biolimus A9 and Sirolimus, have strong ability to reduce neointima proliferation and need for target lesion revascularisation. In this study, as in previous studies with Nobori stent, there were no cases of late stent thrombosis. However, at 12 months only five patients were not on dual antiplatelet therapy and it remains to be seen at further follow-up whether the low MACE rate and stent thrombosis can be maintained after discontinuation of this combined therapy. Prescription of dual antiplatelet therapy far beyond per protocol mandated period probably reflects the latest recommendations.18
Study limitation
This study has several limitations, the main ones being the small number of patients, lack of direct randomisation, relatively low compliance with angiographic follow-up and selection of angiographic rather than clinical primary endpoint. Nonetheless, we believe that the data generated by enrolling patients under less restrictive exclusion criteria and planned long term follow-up play an important role in establishing safety profile of this new device.
Conclusions
Based on the nine months angiographic results of NOBORI CORE study it is reasonable to conclude that the Nobori stent is effective in reducing neointima formation and that similarity to Cypher stent could be proven. Low MACE rate at 12 months confirms good safety profile of both stents for the type of patients as enrolled in this study. Larger, randomised trials, sufficiently powered to assess clinical endpoints, will be needed to assess possible beneficial effects of biodegradable polymer on long term safety.
Acknowledgments
We are grateful to all patients who accepted to participate in this study, to nurses and technicians who took extremely good care of all the aspects of this study. We also acknowledge extraordinary efforts of Toyohisa Shiono and Dr. Claude Dragos who did data analysis and Hirofumi Nagai PhD who gave useful comments during study design and data analysis. The dedication of Dr. Vladimir Borovicanin, Dr. Maja Vuckovic and Marija Markovic and the energy they invested in this study deserve to be mentioned.
Appendix 1
Principal Investigator: Academician Prof. Dr. Miodrag Ostojic, Clinical Centre Serbia, Belgrade, Serbia
Steering Committee: Miodrag Ostojic, Belgrade, Serbia, Dragan Sagic, Belgrade, Serbia, Robert Jung, Sremska Kamenica, Serbia, Milan Nedeljkovic Belgrade, Serbia, Dragica Paunovic, Leuven, Belgium
Clinical Event Committee: Gian Battista Danzi, Ospedale Maggiore Policlinico, Milan, Italy; Philip Urban, Hopital de la Tour, Geneva, Switzerland; Bernhard Reimers, Ospedale di Mirano, Mirano, Italy;
Sponsor: Terumo Europe N.V. Leuven, Belgium
Study sites and investigators:
Belgrade: Clinical Centre Serbia: Miodrag. Ostojic, Branko Beleslin, Milan Nedeljkovic, Sinisa Stojkovic, Dejan Orlic; Institute for Cardiovascular Disease Dedinje: Dragan Sagic, Ljupco Mangovski, Bratislav Milosavljevic, Mirko Colic, Dragan Topic, Zelimir Antonic; Sremska Kamenica: Institute for Cardiovascular Disease: Robert Jung, Dragan Debeljacki, Vladimir Ivanovic, Miroslav Bikicki; Nis: Clinical Centre: Zoran Perisic, Svetlana Apostolovic; Kragujevac: Clinical Centre: Nikola Jagic, Vladimir Miloradovic.

