Colin Berry1, MD PhD; Yoan Lamarche2; Jean-Claude Laborde4, MD; Raymond Cartier2, MD; André Y. Denault3, MD; Arsène Basmadjian3, MD; Raoul Bonan1*, MD
Summary
This report describes the first case of “ad-hoc” simultaneously combined percutaneous aortic valve replacement (PAVR) and coronary artery revascularisation in an 85 year old woman who had been refused aortic valve replacement surgery because of severe co-morbidity. Initial coronary angiography revealed an 80% stenosis of the mid-left anterior descending artery. Percutaneous coronary intervention was performed with deployment of an Endeavor™ drug eluting stent, followed by aortic balloon dilatation and retrograde delivery of a self-expandable CoreValve ReValving™ system. The procedure was successfully completed and the patient was discharged home 11 days later.
Case report
An 85 year-old woman with a history of severe aortic valvular stenosis was refused aortic surgery because of respiratory insufficiency due to bronchiectasis and chronic obstructive pulmonary disease. Her other co-morbid health problems included peripheral vascular disease, atrial fibrillation, hypertension and insulin-treated diabetes mellitus. In 2004, a thallium201 myocardial scintigraphy dobutamine stress test revealed a moderately sized antero-apical reversible perfusion defect. Her medication included coumadin, digoxin, furosemide, pravastatin, metformin, insulin, ipratropium, telmisartan, and diltiazem.
Her functional status was severely limited by dyspnoea (New York Heart Association functional class IV). Pre-operative echocardiography revealed a calcified aortic valve with an area of 0.6 cm2 and a mean gradient of 57 mmHg, consistent with severe aortic stenosis. The left ventricular outflow tract, aortic valve annulus, and ascending aorta diameters were 18 mm, 27 mm, and 34 mm, respectively. There was moderate, concentric left ventricular hypertrophy and the left ventricular ejection fraction was 67%. There was grade 2 mitral and tricuspid regurgitation, the left atrium was mildly dilated (43 mm) and the estimated pulmonary artery systolic pressure was 55 mmHg. Spirometry revealed a forced vital capacity of 1.6 L (86% of predicted), forced expired volume in 1 s of 1.45 L (78% of predicted) and a carbon monoxide lung diffusion capacity transfer factor of 0.41 (21% of predicted). Haematology and renal function were normal.
Following approval for compassionate use by the Montreal Heart Institute Ethics Committee with device availability under special access by the Therapeutic Products Directorate, Ottawa, Canada, the patient was offered percutaneous aortic valve replacement (PAVR) with the CoreValve Revalving™ system (CoreValve Inc, Irvine, CA) in March 2006.
Prior to anaesthesia the patient underwent coronary angiography via the right radial artery. The left coronary artery was dominant and there was an 80% stenosis in the mid-distal segment of its left anterior descending branch. A medico-surgical decision was taken to intervene, and the lesion was first pre-dilated with a 2.5 mm x 15 mm semi-compliant balloon dilatation catheter (Sprinter RX™, Medtronic Inc., Santa Rosa, CA) then treated with a 3 mm x 18 mm drug-eluting stent (Endeavor™, Medtronic, Inc., Santa Rosa, CA) (Figure 1).
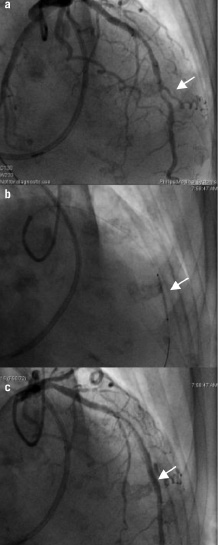
Figure 1. a. Left coronary angiography (RAO 10° CRAN 40°) revealed a tight stenosis (estimated to be 80% of the reference diameter) in the mid-distal left anterior descending artery (LAD; arrow). In addition, angiography revealed non-obstructive disease in the mid-LAD. A Swan-Ganz catheter (Edwards Lifesciences, Irvine, CA) is also evident. b. The LAD stenosis was pre-dilated with a 2.5 mm x 15 mm coronary balloon catheter (Sprinter RX, Medtronic, Inc., MN) at 14 atmospheres and then treated with a 3 mm x 18 mm drug-eluting stent (Endeavor, Medtronic, Inc., MN) which was deployed at 10 atmospheres. c. Non-obstructive plaque in the mid-LAD is evident proximal to the stent which appears optimally deployed (arrow).
After general anaesthesia, aortography was performed followed by aortic valvuloplasty with a 23 mm x 5 cm balloon catheter (Z-MED II™, NuMED Canada Inc., Cornwall, ON). A 26 mm CoreValve Revalving™ system (CoreValve Inc, Irvine, CA) was then delivered in a retrograde fashion from the right femoral artery under left femoro-femoral cardiopulmonary bypass (CPB) with angiographic guidance.
Cardiopulmonary bypass lasted 40 minutes and total blood loss was estimated to be 200 ml. A post-implantation angiogram was obtained (Figure 2) which demonstrated adequate positioning of the device with evidence of trivial valvular regurgitation.
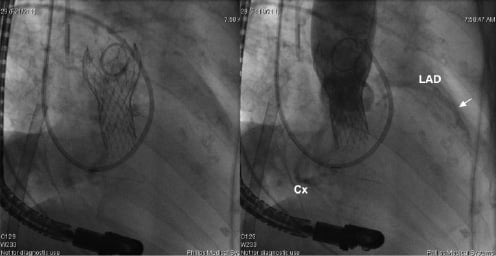
Figure 2. a. Cine acquisition of the CoreValve prosthesis after deployment. A pigtail catheter in the ascending aorta is evident superior to the CoreValve. In addition, a Swan-Ganz catheter (Edwards Lifesciences, Irvine, CA) is present in the right heart and pulmonary artery, an extra-corporeal circulation catheter is located in the right atrium, and a transoesophageal probe is also evident. b. Aortography reveals a well-seated, competent bioprosthesis. The angiogram also shows opacification of the unique left (LCA & Cx) coronary artery with the patent stent (arrow).
Transoesophageal echocardiography demonstrated mean and peak valve gradients of 11 and 18 mmHg, respectively and 2 trace paravalvular leaks. Mitral valve function was unchanged and left ventricular systolic function was well preserved. The patient was weaned uneventfully from ventilation.
Cardiac biomarker concentrations increased, with a peak creatinine kinase MB concentration of 86.0 µg/L (normal range 0-5 µg/L) and a maximum troponin T concentration of 0.6 µg/L (normal range 0-0.03 µg/L). This was associated with new, persistent left bundle branch block. A myocardial perfusion dipyridamole stress test, performed on the sixth post-operative day, revealed normal left ventricular systolic function, a left ventricular ejection fraction superior to 60% and no evidence of ischaemia (Figure 3).
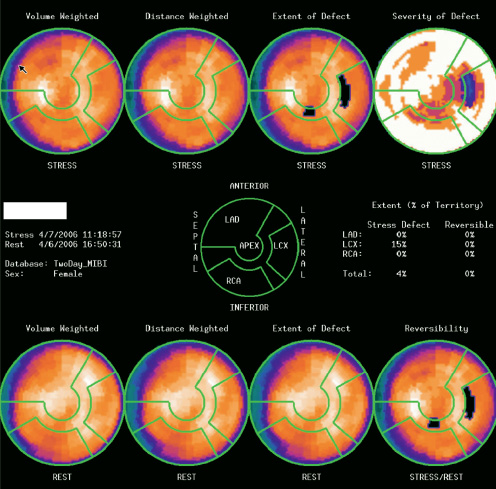
Figure 3. Synchronised myocardial perfusion images obtained at rest and one hour after dipyridamole infusion. The scintigraphic images reveal a uniform radiotracer distribution throughout the myocardium, except for a mild fixed reduction in signal intensity in the anterior wall region best explained by breast attenuation. Left ventricular systolic function was normal with an ejection fraction superior to 60%.
The patient made an otherwise uneventful recovery. At discharge on the eleventh post-operative day, the ultrasound evaluation confirmed the aortic valve area and showed an improvement to less than one trace of regurgitation. Her medication included aspirin 80 mg daily, ramipril 5 mg daily, combivent 2 inhalations 4 times daily, furosemide 40 mg daily, digoxin 0.125 mg daily, warfarin, and clopidogrel 75 mg daily.
Discussion
To the best of our knowledge, we report the first case of “ad-hoc” simultaneously combined coronary artery intervention (PCI) and PAVR following medico-surgical consultation. This report illustrates the potential for percutaneous heart intervention (PHI), incorporating PCI and percutaneous structural heart disease treatment. This case narrows the gap between percutaneous and surgical intervention for acquired structural heart disease1, and underlines the emerging potential of PAVR as an alternative to palliative medical therapy in subjects unsuitable for conventional surgery.
Percutaneous aortic valve replacement is a new technique, first described in 2002 by Cribier A. et al2 Two retrograde PAVR cases with the CoreValve SystemTM (CoreValve Inc., Irvine, CA),3,4 which differs in several respects to PAVR with the Cribier-Edwards valve (Edwards Lifesciences Inc.),5,6 have already been described. We are aware that some patients have undergone PCI as a staged procedure, during screening for PAVR with the Cribier-Edwards percutaneous valve (Edwards Lifesciences Inc. Irvine, CA)5,6. We also understand that in one recent CoreValve PAVR case, acute occlusion of the left anterior descending coronary artery occurred shortly after PAVR and emergent PCI was performed7.
An optimal screening approach for PAVR should involve invasive and non-invasive assessments. Non-invasive screening should include nuclear stress testing for CAD, MRI and/or computed tomographic angiography to assess for abnormalities of the heart and vasculature, and transthoracic echocardiography to assess the heart and aorta. These tests must be complemented by coronary angiography with aortography and in selected cases, transoesophageal echocardiography.
Several factors should be considered in patients referred for PAVR. The patient should be carefully evaluated for the presence of ischaemia prior to PAVR. Coronary angiography should be performed as part of an elective screening process. If obstructive coronary artery disease is demonstrated, consideration should be given to the feasibility and timing of PCI in relation to PAVR. Consideration should also be given to the PCI approach (single vs. staged procedure), technical factors (e.g. type of stent – bare metal vs. drug eluting), and risks of the PCI procedure (e.g. potential for haemodynamic instability, contrast volume, risk of stent thrombosis). Furthermore, the question of thienopyridine therapy, and in particular, its duration, should also be considered. PCI combined with PAVR, where stenting is performed prior to extra-corporeal circulation and prosthetic valve implantation, is probably associated with a higher risk of acute stent thrombosis. CPB is a low-flow pressure system, and reduced antegrade coronary flow predisposes to acute stent thrombosis after PCI.
We recommend an individualised approach based upon an estimated risk/benefit analysis, rather than a common strategy. Coronary anatomy, such as in the case of left main stem stenosis, may determine that PCI be performed first followed by PAVR at a later time. Alternatively, in the case of symptomatic aortic stenosis with less advanced coronary artery disease – e.g. distal right and/or circumflex, it may be more appropriate to first correct the aortic stenosis followed by PCI. Deferred PCI may have advantages and disadvantages. Advantages of deferred PCI after correction of obstructive aortic valve disease include a reduced risk of haemodynamic instability and, consequently, acute closure and stent thrombosis. In some cases, medical therapy may be preferred to PCI for management of angina.
Our initial screening strategy employed elective non-invasive investigations because of the frail nature of our PAVR referrals. Consequently, coronary angiography was not performed prior to PAVR in the present case. In one other female patient, non-invasive magnetic resonance angiography overestimated the left iliac artery lumen diameter (7 mm) compared with the measurement obtained by invasive X ray angiography at the time of PAVR (6 mm). This discrepancy contributed to our failure to advance the delivery catheter across the aorto-iliac junction and the procedure was safely terminated. Based on these experiences, we now perform coronary angiography and aortography prior to PAVR.
We detected a rise in cardiac biomarkers post-PAVR associated with new left bundle branch block. These abnormalities may be explained by a complication either due to PCI or PAVR. Contemporary guidelines assert that cardiac biomarker elevation is only likely to occur in patients with symptoms or signs of MI after percutaneous coronary intervention (PCI) or in whom PCI was complicated8. In keeping with this position, we believe that biomarker elevation following PAVR in this case may be more readily attributed to mechanical injury than to ischaemia related to PCI, which was straightforward. Myocardial injury during “revalving” may have occurred during pre- valve placement balloon valvuloplasty as multiple inflations were necessary because of balloon slippage during inflation. Myocardial damage may also have occurred as a consequence of prosthetic valve deployment. This hypothesis is supported by the normal dipyridamole myocardial perfusion stress test performed on the sixth post-operative day (Figure 3). The nuclear stress test demonstrated normal systolic function without evidence of ischaemia or infarction, which effectively excludes device-related transient or complete coronary artery occlusion. We propose an alternative explanation for the post-PHI cardiac biomarker elevation which is direct myocardial injury due to multiple balloon inflations during the valvuloplasty, and device positioning and expansion in the out-flow tract of the left ventricle. This form of myocardial injury may have less prognostic significance than myocardial injury due to coronary artery disease.
In conclusion, this case may represent a new treatment paradigm for patients with severe symptomatic aortic valve disease and coronary artery disease who are unsuitable for surgery. We recommend at this stage an individualised approach to the timing and treatment selection of PHI.
Acknowledgement
Dr Berry previously received Fellowship support from Medtronic Inc. (Europe), and is currently funded by a British Heart Foundation International Fellowship.