Abstract
Aims: To assess major bleeding complications in patients after coronary stenting who are on dual antiplatelet drugs with or without oral anticoagulants.
Methods and results: Bleeding complications necessitating hospital admission were prospectively recorded during initial hospitalisation and during a complete follow-up of three years in 813 consecutive patients undergoing coronary artery stenting. All patients were assigned to antiplatelet therapy with aspirin and clopidogrel for at least six months and continued aspirin use thereafter. There were 25 early bleedings and 26 late hospital admissions for bleeding. Forty-four patients (5.4%) were on oral anticoagulants (coumadin) in addition to antiplatelet agents. The rate of late severe bleeding was 6.1% per year with, vs. 0.8% without coumadin (p <0.0001). In multivariate analyses GPIIb/IIIa (OR 3.8 95%-CI 1.6-8.8, p=0.002), female gender (=R 2.5, 95%CI 1.1-5.8, p=0.04) and age (OR 1.44 per decade, 95%CI 0.99-2.08, p=0.05) were independent predictors of early bleeding; and LVEF (OR 0.65 per 10% increase, 95%CI 0.48-0.87, p=0.004), history of malignancy (OR 5.1, 95%CI 1.5-17.0, p=0.009) and coumadin use (OR 3.5,95%CI 1.1-11.5, p=0.04) for late bleeding.
Conclusions: For patients on oral anticoagulants, drug eluting stents necessitating sustained dual antiplatelet therapy should be used with caution. Specific risk factors for bleeding complications should guide anticoagulation after PCI.
Abbreviations
BASKET: BAsel Stent Kosten Effektivitäts Trial
BMS: bare metal stent
DES: drug eluting stent
PCI: percutaneous coronary intervention
LVEF: left ventricular ejection fraction
NSAID: non-steroidal anti-inflammatory drug
OR: odds ratio
Introduction
Percutaneous coronary intervention (PCI) with stent placement is increasingly used in the elderly population1-3. Therefore, a growing number of patients with indications for oral anticoagulants have to be treated simultaneously with antiplatelet therapy3. However, in such patients, the bleeding risk may be increased up to 5-fold as evidenced by case reports and retrospective analyses5-11. Still, there are no prospective data available to determine the true incidence of bleeding complications after PCI in patients with such combined antithrombotic therapy, since patients requiring coumadin therapy usually are excluded from randomised trials12-15.
Therefore, we assessed the incidence of clinically significant bleeding complications depending on the antithrombotic regimen, and identified risk factors for early and late bleeding complications after PCI using the BASKET (BAsel Stent Kosten Effektivität Trial) database16,17.
Methods
BASKET was a randomised controlled trial enrolling 826 consecutive patients undergoing PCI17. The study protocol was approved by the local ethics committee and all participants gave written informed consent. According to the BASKET protocol, patients received either bare-metal (BMS) or drug eluting stents (DES) in a 1:2 randomised fashion, irrespective of the indication. The only exclusion criteria were: in-stent restenosis, large vessel PCI (>4.0 mm), since not all stents were available in this size at that time; and lack of informed consent. Femoral access sites were used in all patients with sheath sizes mostly 6 Fr and, exceptionally, 7 Fr. Patients were on dual antiplatelet therapy (acetylsalicylic acid [aspirin] 100 mg and clopidogrel 75 mg per day, after a single loading dose of 300 mg) for at least six months after PCI according to the guidelines at that time18. After the first six months, aspirin was continued in all patients. The use of glycoprotein IIb/IIIa antagonists and heparin during or immediately after the procedure was at the discretion of the operator. Follow-up contacts were made at 6, 18, and 36 months as described16.
For this analysis only major bleedings were considered since minor bleedings may have remained unrecorded. Bleeding was defined as major if it required a therapeutic intervention (surgery or blood transfusion), if there was a fall of at least 2g% in haemoglobin concentration, or if it was the cause for hospital admission or resulted in death, according to previously described definitions19. Bleeding complications were defined as early if they occurred during, and late if they occurred after, the index hospitalisation.
Statistical analysis
Values are expressed as frequencies, mean±standard deviation (SD), or median with the interquartile range (IQR), as appropriate. χ2-test, Fisher’s exact test, t-test, or Mann-Whitney U-test were used for group comparisons, as appropriate. Univariate logistic regression was used for calculation of odds ratios (OR) for total, early, and late bleedings. Multivariate logistic regression using stepwise backward method was performed to seek independent predictors of total, early, and late bleeding including those variables showing an association with bleeding at a p-level of 0.10 in univariate regression analysis. We used no time dependent analysis, since follow-up was almost complete in all patients16. Still, time-dependent analysis did not influence results significantly (data not shown). SPSS package software version 15.0 was used for statistical calculations. A two-sided p-value of <0.05 was considered to be statistically significant.
Results
Patients
From the 826 BASKET patients, four were excluded due to insufficient data regarding precise anticoagulation therapy and nine because they were on antithrombotic monotherapy throughout the three year study period for specific reasons. Thus, 813 patients were included in this analysis, with 44 patients (5.4%) on oral anticoagulation (Figure 1).
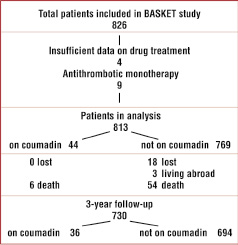
Figure 1. Flow chart of the study population.
Baseline characteristics
Baseline characteristics of patients with and without oral anticoagulation are given in Table 1.
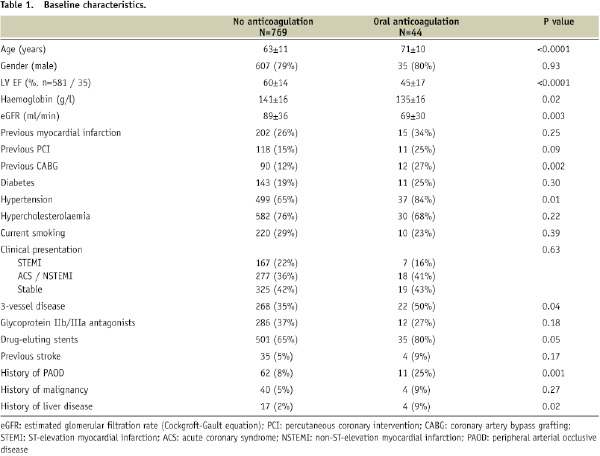
Patients receiving oral anticoagulation were older, more often had previous coronary bypass surgery and had more comorbidities, a lower left-ventricular ejection fraction (LVEF) and worse renal function than patients without oral anticoagulation.
Bleeding complications
Bleeding complications during the entire 3-year follow-up as defined for this study occurred in a total of 50 of the 813 patients (6.2%). These were early bleedings in 25 (3.1%), late bleedings in 26 patients (3.2%). In one patient, there was both an early and a late bleeding. Types of early and late bleedings are summarised in Table 2.
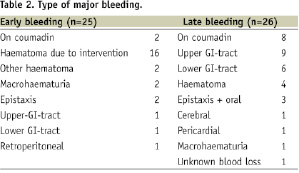
Most early bleedings were directly related to the intervention. Late bleedings occurred after a median of 232 days (interquartile range 145–699 days).
Risk factors for total, early and late bleedings in univariate analysis are depicted in Table 3.
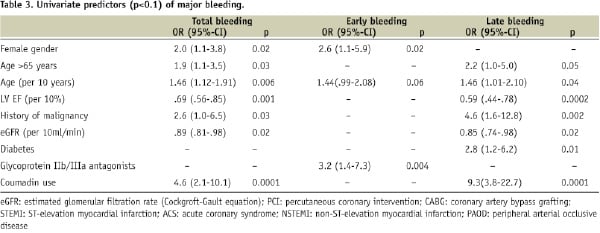
The most important risk factor was the antithrombotic regimen. The use of glycoprotein IIb/IIIa antagonists was associated with an increased risk of early bleedings (5.4% versus 1.7%, odds ratio [OR]=3.2, 95%-CI 1.4-7.3, p=0.006), whereas total bleedings (7.7% versus 5.2%, OR=1.5, 95%-CI 0.85-2.7, p=0.17) and late bleedings (2.7% versus 3.5%, OR=0.76, 95%-CI 0.33-1.8, p=0.68) were not influenced (Figure 2).
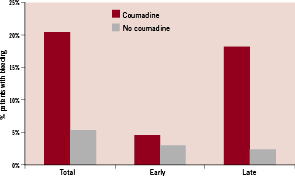
Figure 2. Percentage of total (p=0.001), early (p=0.64) and late bleeding complications (p<0.0001) during three years of follow-up in patients taking (n=44) and not taking coumadin (n=769).
The number of patients on combined antithrombotic treatment with coumadin and antiplatelet agents was 44 (5.4%). In these patients, the total bleeding rate was considerably higher than in those without (OR=4.6, 95%-CI 2.1-10.1), resulting mainly from late bleedings (OR=9.3, 95%-CI 3.8-22.8), whereas the early bleeding did not differ significantly between the two groups (OR=1.5, 95%-CI 0.35-6.8). The annual rate of late bleedings was 6.1% in patients taking coumadin, whereas this was only 0.8% in those not taking coumadin (p <0.0001).
Renal failure and age were other risk factors for both early and late bleedings. Women were at increased risk of early bleedings. Late bleedings were additionally related to reduced LVEF, history of malignancies, and diabetes. A history of hypertension, bleeding, gastric ulcer or cerebrovascular accidents prior to PCI or the use of NSAID had no influence on bleeding risk. Also, the type and number of coronary lesions treated, number and type of stents used and presentation at baseline did not influence bleeding risk.
The results of multivariate analyses are presented in Table 4.
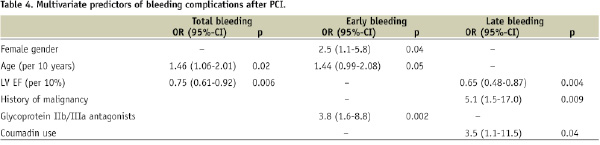
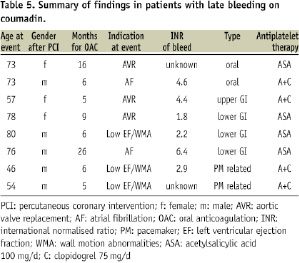
Independent predictors of early bleeding were glycoprotein IIb/IIIa antagonist use, female gender and age. Independent predictors of late bleeding were LVEF, history of malignancy and coumadin use. A summary of detailed characteristics in patients with late bleedings on coumadin is given in Table 5.
Since LVEF was not known in all patients (known in n=616 or 76%; unknown mostly in those patients with acute coronary syndrome), multivariate logistic regression analysis was performed twice, including and excluding LVEF for late bleeding.
Excluding LVEF, again coumadin use (OR=8.7, 95%-CI 3.5-21.9, p<0.0001) and a history of malignancy (OR=3.9, 95%-CI 1.3-11.4, p=0.02) and in addition diabetes (OR=2.5, 95%-CI 1.1-5.7, p=0.04), were independent predictors of late bleeding.
Discussion
Our data show that in an unselected patient population followed over three years the use of combined antiplatelet and anticoagulant therapy was necessary only in a small minority of patients, but was associated with a substantial risk of late bleedings after PCI and stenting. Whereas, in patients without oral anticoagulation the risk of clinically relevant bleedings was low (<1% per year during late follow-up), the use of coumadin increased the risk by a factor of eight. Also, the risk of early bleedings was associated with the peri-interventional use of glycoprotein IIb/IIIa antagonists.
The risk of bleeding with combined antiplatelet and anticoagulant therapy has been reported so far only from observational studies9,20, from selected patient subgroups5,21, from retrospective data6,22 and from hospital studies with no long-term follow-up23,24. Direct comparison with our data is difficult, mainly because of the different patient selection and duration of follow-up in these studies.
The highest bleeding rate was reported in a study by Manzano et al5. These authors found a rate as high as 27.4% for major bleedings during 12 months follow-up in 51 patients with atrial fibrillation who received coronary stents and who were treated with dual antiplatelet therapy plus oral anticoagulants. The rate of bleeding in these patients was much higher than in our population. However, the rate contrasts also with other previous reports as discussed below. This difference may be explained at least in part by patient selection in their study which resulted in a generally sicker population. In particular, these patients were older and had a higher prevalence of diabetes and renal failure, which have been identified as risk factors for bleeding in our cohort.
In the case control study of Rossini11, a very similar proportion of the entire PCI population was on oral anticoagulants (7%). Although during a follow-up of 18 months they reported rates of early (1%) and late (3%) major bleedings in patients on triple therapy, which is comparable to our data, this represented only a non-significant increase when compared to the control group. The controls, however, were not selected prospectively, and no data are reported on renal function and malignancy which were two risk factors in our study.
In the study by Sarafoff et al25 in 515 patients undergoing stent implantation while on oral anticoagulant therapy (mostly for atrial fibrillation), the rate of major bleedings was only 2.1% and there was no difference between the subgroups on dual or triple antithrombotic therapy. However, patients with malignancies were excluded, which may be one reason for a lower rate of bleeding. In addition, a direct comparison of these data is difficult due to a short follow-up of only 12 weeks. Similarly Ruiz et al22, in their study of stenting in 426 patients with atrial fibrillation, found a bleeding rate of 12.3% during one and a half years of follow-up, with no difference regarding the use of coumadin in addition to antiplatelet therapy. The lack of an influence of additional coumadin use on bleeding risk in this study also contrasts with the findings of our study. Again, patients in this study were selected on the basis of atrial fibrillation and their risk profile was different regarding age, prevalence of diabetes and previous ischaemic or embolic events. In addition mortality during follow-up was high which could have hampered the emergence of bleeding complications due to competing risks. Lately, Karjalainen et al6, in their study of 239 patients on anticoagulants, reported a rate of 8.2% major bleedings at 12 months follow-up, which is comparable with our results, risk factors for bleeding being the use of glycoprotein receptor antagonists, female gender and smoking.
In the present analysis, we were able to identify some distinct circumstances which increase the risk of bleeding. The use of glycoprotein receptor antagonists was associated with a significantly higher risk of early bleeding after PCI, whereas it had no impact on total bleeding rate. This is in accord with the study by Manzano5. Bleeding rates have been reported to increase with age alone also in other settings of antithrombotic therapy21. Further risk factors in our study were female gender, history of malignancy, low LV ejection fraction and renal failure. Low LVEF, with wall motion abnormalities, was an indication for oral anticoagulants in three of the patients with late bleeding.
These factors have previously been identified to increase bleeding risk in some studies6. In studies of anticoagulant use in atrial fibrillation, it is known that some of the CHADS risk factors (heart failure, hypertension, age, diabetes and stroke) do not only increase the risk for stroke, but also for haemorrhagic complications26.
It may be argued that the bleeding rate observed in our patients on coumadin was caused solely by this drug regardless of concomitant antiplatelet use. However, patient populations treated with coumadin alone show rates below 1% per year27.
Drug-eluting stents necessitate treatment with both aspirin and clopidogrel for at least one year, according to current guidelines4. This treatment is increasingly used also in elderly patients who may have an indication for oral anticoagulants. Based on the findings of this study, the potential risk of embolic complications must be carefully balanced against the risk of major bleeding. Identification of individual risk factors for both embolic and bleeding events may help to decide if coumadin should be discontinued or if bare-metal stents necessitating dual antiplatelet therapy for a few weeks only should be preferred. Data from the BASKET trial indeed suggest that the use of bare-metal stents is of equal benefit than coated stents in larger vessels28. In some patients, coumadin may be withheld if risk factors for bleeding are present, at least as long as the use of dual antiplatelet therapy is required.
Limitations
Although patients were prospectively included and followed in the study, this is a retrospective analysis. Also, indications for oral anticoagulants (atrial fibrillation, valve prostheses or severely reduced LVEF) have not been analysed in detail in all patients and quality of INR- control is unknown in some patients.
The absolute number of events (51) is limited, and therefore the analysis has a low statistical power. Nevertheless, we were able to determine several independent variables of increased risk.
Conclusions
The present study of patients on single or dual antiplatelet therapy after PCI demonstrates a rate of bleeding eight times higher if additional coumadin was used when compared to patients on antiplatelet therapy alone. Other independent risk factors for bleeding complications during long-term follow-up were diabetes, low LVEF, and history of malignancy. Based on these findings, it may be advisable to use DES with some caution in patients on oral anticoagulants in order to avoid prolonged periods of triple antithrombotic therapy.
In addition, it seems necessary to carefully balance risks and benefits of additional coumadin anticoagulants, at least for the duration of aggressive dual antiplatelet therapy in individual patients, particularly if they have additional risk factors for bleeding and not an excessive risk of embolic events.

