Abstract
Aims: Restenosis is higher among certain subpopulations when subjected to percutaneous coronary interventions even when using drug-eluting stents. The randomised SPIRIT II trial demonstrated the superiority of the XIENCE V™ Everolimus Eluting Coronary Stent System over the TAXUS™ Paclitaxel-Eluting Stent System in terms of in-stent late loss at six months among 300 patients treated for de novo native coronary artery lesions.
Methods and results: In this post-hoc analysis of SPIRIT II we focused on six-month angiographic outcomes of diabetic patients (n=69), left anterior descending arteries (n=149), long lesions >20 mm (n=43), small vessels <3.0 mm (n=209) and type B2 and C lesions (n=233). In-stent late loss was consistently less among all subgroups when treated by everolimus-eluting stents compared to paclitaxel-eluting stents: diabetics 0.15±0.26 mm versus 0.39±0.34 mm, p=0.006; LAD 0.12±0.23 mm versus 0.44±0.37 mm, p<0.001; long lesions 0.13±0.26 mm versus 0.43±0.46 mm, p=0.070; small vessels 0.17±0.28 mm versus 0.37±0.39 mm, p<0.001; B2/C lesions 0.12±0.31 mm versus 0.36±0.36 mm, p<0.001.
Conclusion: The everolimus-eluting stent remained superior in terms of in-stent late loss in a variety of higher risk populations for restenosis compared to the paclitaxel-eluting stent. These analyses were consistent with the in-stent late loss results of the overall SPIRIT II trial population.
Introduction
Recent studies that have evaluated the local application of anti-proliferative drugs (sirolimus and paclitaxel) for the prevention of restenosis via a stent delivery system have shown that these therapies successfully inhibit the development of neointimal hyperplasia and reduce restenosis and associated clinical events.1,2
The feasibility of using everolimus on a drug-eluting stent was demonstrated in the earlier FUTURE-I3,4 and FUTURE II5,6 studies and more recently in the SPIRIT FIRST7 study, using the everolimus-eluting stent. The SPIRIT II trial8 was a continuation of the assessment of the safety and performance of the XIENCE V everolimus eluting coronary stent versus the TAXUS paclitaxel-eluting coronary stent in the treatment of patients with a maximum of two de novo native coronary artery lesions. SPIRIT II has met its primary endpoint, namely it showed an in-stent late loss in the everolimus arm, which was not only non-inferior but also superior to the in-stent late loss observed in the paclitaxel arm at six months (0.11±0.27 mm versus 0.36±0.39 mm, respectively p<0.0001). Per protocol, the overall study cohort was a low-risk population for neointimal hyperplasia and restenosis; focusing on the higher risk subgroups is therefore of particular interest. This sub-analysis of SPIRIT II trial is directed to the following subsets: diabetic patients, lesions located in the left anterior descending artery (LAD), long lesions > 20 mm, lesions in small vessels < 3.0 mm in diameter and type B2 and C lesions according to the modified American College of Cardiology/American Heart Association (ACC/AHA) classification system for lesion morphology.9,10
Methods
Patients and design
Details of SPIRIT II and the XIENCE V stent have been recently described.8 In brief, this prospective, randomised (3:1) single-blind, parallel two-arm trial was performed at 28 centres in Europe, India and New Zealand and enrolled patients from July 2005 to November 2005. 300 patients were included of which 223 were randomly assigned to the everolimus-eluting stent and 77 to the paclitaxel-eluting stent. It was approved by the ethics committee at each participating institution, and all patients gave written informed consent.
Patients were eligible for the study if they were older than 18 years and had evidence of myocardial ischaemia. The patient could have a maximum of two de novo native coronary artery lesions, which had to be located in different major epicardial vessels. The de novo target lesion(s) had to have a reference vessel diameter between 2.5 mm and 4.25 mm by visual estimation, a target lesion length < 28 mm, a visually estimated stenosis between 50-99% of the luminal diameter, and a Thrombolysis In Myocardial Infarction (TIMI) flow grade of 1 or more. Patients were not eligible for enrolment if they had known diagnosis of acute myocardial infarction three days prior to the baseline procedure, a left ventricular ejection fraction of less than 30%, or were awaiting a heart transplant. Additionally, patients having target lesion(s) with an aorto-ostial or left main location, a lesion located within 2 mm of the origin of the left anterior descending or left circumflex, heavy calcification, or a visible thrombus within the target vessel were also excluded from the trial.
In this substudy of SPIRIT II the following subgroups were investigated: diabetic patients, lesions located in the left anterior descending artery (LAD), long lesions > 20 mm, lesions in small vessels < 3.0 mm in diameter and type B2 and C lesions. All are post-hoc analysis subgroups (i.e. not prespecified in the study protocol). The main analysis in this substudy was to compare both stent treatment groups among the different higher risk populations. A secondary analysis compares each specific higher risk subgroup to the remaining study population for each treatment group.
The Everolimus-Eluting Stent
The XIENCE V Everolimus Eluting CSS (Abbott Cardiovascular Systems, Abbott Park, IL, USA) is comprised of the ACS MULTI-LINK VISION stent and delivery system, and a drug-eluting coating. The ACS MULTI-LINK VISION Stent is a balloon expandable stent, which consists of serpentine rings connected by links fabricated from a single piece of medical grade L-605 cobalt chromium alloy.
Study procedure
Following the confirmation of angiographic in- and exclusion criteria prior to the procedure, patients were allocated through a telephone randomisation service and assigned in a 3:1 ratio to either an everolimus-eluting stent or a paclitaxel-eluting stent. The XIENCE V stents were available in lengths of 8, 18 and 28 mm, and diameters of 2.5, 3.0, 3.5 and 4.0 mm. Lesion lengths between 22 and 28 mm had to be covered with 2 stents in the XIENCE V group, twice a 18 mm stent, or a 28 mm and a 8 mm stent.
Lesions were treated using standard interventional techniques with mandatory pre-dilatation and stent implantation at a pressure not exceeding the burst pressure rate. Due to packaging differences, physicians were not blinded to the device. Post-dilatation was left to the discretion of the physician, however, if performed, was only to be done with balloons sized to fit within the boundaries of the stent. In the event of a bailout procedure and additional stent requirement, the stent had to be one from the same group as the first implanted stent. Patient preparation and pharmaceutical treatment during the procedure were to be in accordance with standard hospital practice. The use of GPIIb/IIIa inhibitors was left to the discretion of the physician.
Follow-up
Patients were evaluated at 30, 180, 270 days and one year. Further evaluations will be performed at two, three, four and five year(s). All patients were to receive 75 mg clopidogrel for a minimum of 180 days and > 75 mg aspirin for a minimum of one year. At outpatient visits, patients were asked specific questions about the interim development of angina or the occurrence of adverse events. In this substudy we focus only on the angiographic follow-up performed at 180 days.
Quantitative Coronary Angiography evaluation
Quantitative coronary angiography (QCA) was performed using the CAAS II analysis system (Pie Medical BV, Maastricht, Netherlands). In each patient, the stented segment and the peri-stent segments (defined by a length of 5 mm proximal and distal to the stent edge) were analysed. The following QCA parameters were computed: minimal luminal diameter (MLD), reference diameter obtained by an interpolated method, and percentage diameter stenosis. Binary restenosis was defined in every segment as diameter stenosis >50% at follow-up. Late loss was defined as the difference between MLD post-procedure and MLD at follow-up.
Study endpoints
The primary angiographic endpoint in the main study was in-stent late loss at 180 days, as determined by quantitative angiography, based on an “analysis lesion”: one randomly selected lesion per patient to avoid inter-lesion dependence.11 Secondary endpoints (QCA) included the in-segment late loss and in-stent and in-segment angiographic binary restenosis rate analysed on all lesions available. In this paper all lesion results are reported.
In-stent was defined as within the margins of the stent while in-segment was defined as located within the margins of the stent and 5 mm proximal or distal to the stent. Late loss was calculated as the difference between the post-procedure and follow-up minimum luminal diameters.
Statistical analysis
In this paper continuous variables, are expressed as mean± standard deviation. 95% confidence intervals of the difference are estimated by Gaussian approximation. P-values are obtained by using a two-sided Wilcoxon rank-sum test (ITT, alpha 0.05). For binary variables, percentages are presented and p-values are obtained by using a two-sided Fisher exact test (ITT, alpha 0.05). Interaction tests were based on generalised linear models. Subgroup analyses include all lesions.
Caution must be exercised when interpreting p-values displayed for analyses other than those performed for the primary endpoint in the overall population, as none of the other analyses were pre-planned and the study was not powered to detect differences on any of those other variables or subgroups. The resulting p-values, whether or not less than 0.05, may be a result of pure chance.
Results
Diabetic subgroup
Sixty-nine patients (23%) of the whole study population (n=300) were diabetic; 51 patients (23%) of the everolimus-eluting stent group, and 18 (24%) of the paclitaxel-eluting stent group. Baseline clinical and angiographic characteristics were comparable except for more current tobacco use, with fewer smokers in the everolimus group (Table 1).
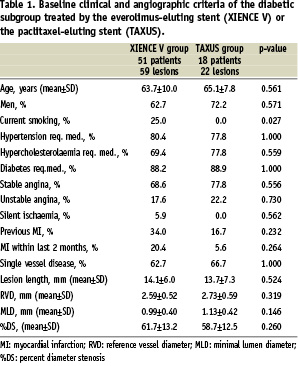
Angiographic outcome measures at follow-up are shown in Table 6.
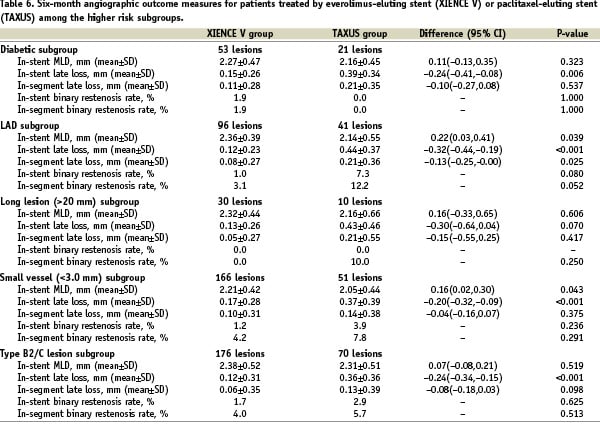
At six months the in-stent late loss was 0.15±0.26 mm in the everolimus arm versus 0.39±0.34 mm in the paclitaxel arm (difference: –0.24 mm [95% CI= –0.41 mm, –0.08 mm] p=0.006). The results of the secondary analysis which compares the diabetic subgroup to the remaining study population for each treatment group are shown in Table 7.
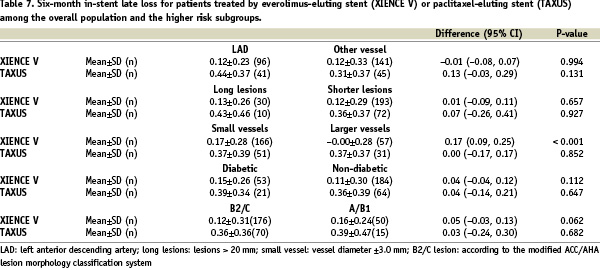
Although non-significantly different, in-stent late loss was higher for both stents among the diabetic population compared to non-diabetics: for the everolimus-eluting stent it was 0.15±0.26 mm for the diabetic group versus 0.11±0.30 mm for non-diabetic group; difference 0.04 [95% CI –0.04, 0.12] p=0.11. For the paclitaxel-eluting stent it was 0.39±0.34 mm among the diabetic group compared to 0.36± 0.39 mm among non-diabetics; difference 0.04 [95% CI –0.14, 0.21] p=0.65.
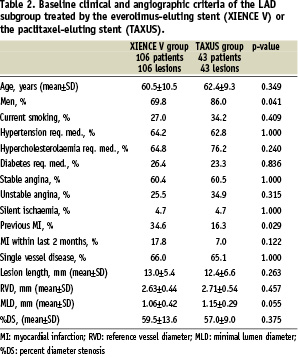
LAD subgroup
One-hundred and forty-nine (149) SPIRIT II patients (49.7%) were treated for LAD stenoses; 106 patients (47.5%) of the everolimus group and 43 patients (55.8%) of the paclitaxel group. Baseline clinical and angiographic characteristics were comparable except for fewer men and more prior myocardial infarction in the everolimus group (Table 2).
Angiographic outcome measures at follow-up are shown in Table 6. At six months the in-stent late loss was 0.12±0.23 mm in the everolimus arm versus 0.44±0.37 mm in the paclitaxel arm (difference: –0.32 mm [95% CI= –0.44 mm, –0.19 mm] p<0.001). The results of the secondary analysis which compares the LAD subgroup to the remaining study population for each treatment group are shown in Table 7. In the everolimus arm, the in-stent late loss was 0.12±0.23 mm versus 01.2±0.33 mm when implanted for LAD stenoses compared to non-LAD lesions respectively (difference –0.01 [95% CI –0.08, 0.07]). Although non-significantly different, in the paclitaxel arm, the mean late loss was 0.44±0.37mm for the LAD population compared to 0.31±0.37mm non-LAD (difference 0.13 [95% CI –0.03, 0.29]).
Long lesion subgroup
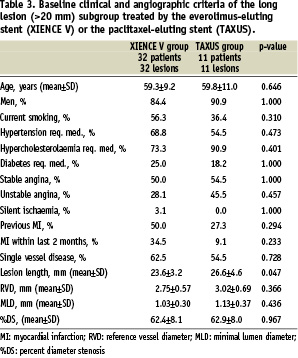
Forty-three SPIRIT II patients (14.3%) were treated for lesions > 20 mm (and had lesion length assessable by QCA); 32 patients (14.3%) of the everolimus group and 11 patients (14.3%) of the paclitaxel group. Baseline clinical and angiographic characteristics were comparable except for lesion length which was longer in the paclitaxel group for this long lesions subgroup (Table 3).
The mean lesion length was 23.59±3.17 mm for the everolimus group and 26.62±4.59 mm in the paclitaxel group (difference –3.03; 95% CI –6.25, 018). Angiographic outcome measures at follow-up are shown in Table 6. At six months the in-stent late loss was 0.13±0.26 mm in the everolimus arm versus 0.43±0.46 mm in the paclitaxel arm (difference: –0.30 mm [95% CI= –0.64 mm, 0.04 mm] p=0.07). However the very limited number of lesions in this subgroup limits the validity of any conclusion. The results of the secondary analysis which compares the long lesion subgroup to the remaining study population for each treatment group are shown in Table 7. In this study the long lesions had a non-significantly greater late loss in both stent arms compared to lesions < 20 mm: 0.13±0.26 mm versus 0.12±0.29 mm; difference 0.01 [95% CI –0.09, 0.11] p=0.66 for the everolimus stent and 0.43±0.46 mm versus 0.36±0.37 mm; difference 0.07 [95% CI –0.26, 0.41] p=0.93 for the paclitaxel-eluting stent. However the very limited number of lesions in the long lesions subgroup limits the possibility to draw any conclusion.
Small vessel subgroup
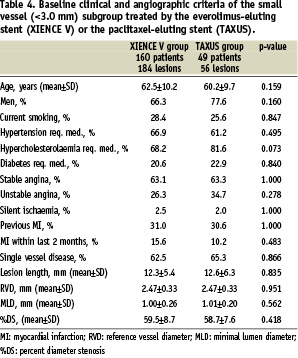
Two-hundred and nine (209) SPIRIT II patients (69.7%) were treated for lesions in vessels <3.0 mm in diameter (and had reference diameter assessable by QCA); 160 patients (71.7%) of the everolimus group and 49 patients (63.6%) of the paclitaxel group. Baseline clinical and angiographic characteristics were comparable with no significant differences between both groups (Table 4).
The mean pre-procedure reference vessel diameter was 2.47±0.33 mm in both arms. Angiographic outcome measures at follow-up are shown in Table 6. At six months the in-stent late loss was 0.17±0.28 mm in the everolimus arm versus 0.37±0.39 mm in the paclitaxel arm (difference: –0.20 mm [95% CI= –0.32 mm, –0.09 mm] p<0.001). The results of the secondary analysis which compares the small vessel subgroup to the remaining study population for each treatment group are shown in Table 7. When comparing patients treated with the everolimus-eluting stent for vessels < 3.0 mm in diameter with those > 3.0 mm, there was a significant difference in in-stent late loss at 6-months between both groups. (0.17±0.28 mm (n=166) versus –0.00±0.28 mm (n=57) respectively; difference 0.17 mm [95% CI 0.09, 0.25] p < 0.001). This difference was not observed in the paclitaxel-eluting stent groups.
B2 and C type lesion subgroup
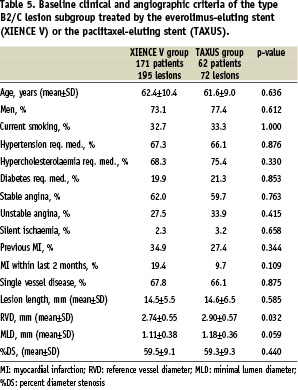
Two-hundred and thirty-three (233) SPIRIT II patients (77.6%) were treated for type B2 or C lesions (and had lesion type assessable by angiography); 171 patients (76.7%) of the everolimus group and 62 patients (80.5%) of the paclitaxel group. Baseline clinical and angiographic characteristics were comparable except for smaller reference diameter in the everolimus arm (Table 5).
Angiographic outcome measures at follow-up are shown in Table 6. At six months the in-stent late loss was 0.12±0.31 mm in the everolimus arm versus 0.36±0.36 mm in the paclitaxel arm (difference: –0.24 mm [95% CI=–0.34 mm, –0.15 mm] p<0.001). The results of the secondary analysis which compares the B2/C type lesion subgroup to the remaining study population for each treatment group are shown in Table 7. In this study in-stent late loss for the everolimus-eluting stent amongst type B2/C lesions was 0.12±0.31 mm compared to 0.16±0.24 mm among type A/B1 lesions (difference 0.05 [95% CI –0.03, 0.13] p=0.062).
Figure 1 compares six-month in-stent late loss for patients treated by everolimus-eluting stent or paclitaxel-eluting stent among the overall population and the higher risk subgroup.
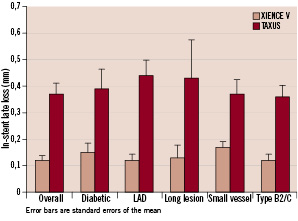
Figure 1. Six-month in-stent late loss for patients treated by everolimus-eluting stent (XIENCE V) or paclitaxel-eluting stent (TAXUS) among the overall population and the higher risk subgroups.
Discussion
The main finding of this differentiated analysis is the consistent trend for superiority of the XIENCE V everolimus-eluting coronary stent over the TAXUS paclitaxel-eluting coronary stent in terms of late loss reduction among diabetic patients, the LAD population, small vessels <3.0 mm, long lesions > 20 mm and type B2 and C lesions as was reported for the whole population treated in the SPIRIT II Trial.
Involvement of the left anterior descending artery is considered an independent risk factor for restenosis after balloon angioplasty and after stent implantation by current guidelines.12 Surprisingly, lesions located in the LAD were shown to have a decreased restenosis rate among complex patients treated with sirolimus-eluting stents.13 It was postulated whether the LAD location represents a true protective characteristic when using sirolimus-eluting stents. In the current analysis everolimus-eluting stents had a similar in-stent late loss when implanted for LAD stenoses compared to non-LAD lesions.
Vessel diameter is an established predictor of angiographic restenosis after catheter-based interventions, with a higher rate of restenosis in smaller vessels.14 In a pooled analysis from FUTURE I and II trials15, the everolimus-eluting stent appeared to be effective in decreasing neointimal proliferation at 6-month follow-up compared with bare-metal stents, across all examined vessel sizes. In those studies the in-stent late loss for the everolimus-eluting stent was 0.19±0.22 mm for vessels <2.75 mm, 0.05±0.23 mm for vessels=2.75-3.25 mm, and 0.14±0.29 mm for vessels >3.25 mm. It was shown for the sirolimus-eluting stent in a subgroup analysis of the RAVEL trial16, that in-stent late loss among vessels <2.36 mm was 0.01±0.25 mm, 0.01±0.38 mm for vessels=2.36-2.84 mm and –0.06±0.35 mm for vessels >2.84 mm.
When comparing patients treated with the everolimus-eluting stent for vessels < 3.0 mm in diameter with those > 3.0 mm, there was a significant difference in in-stent late loss at 6-months between both groups (0.17±0.28 mm versus –0.00±0.28 mm respectively). This difference was not observed among the paclitaxel-eluting stent groups.
Although not being the primary purpose of the American College of Cardiology/American Heart Association (ACC/AHA) classification system for lesion morphology, it was shown17 that this scheme has significant prognostic value after coronary stent placement by being able to influence the restenosis process and thus the entire one-year clinical course of patients. Significant differences were seen mostly between two groups of lesions composed of types A and B1 (simple) and types B2 and C (complex), showing a significant negative impact of lesion complexity on long-term restenosis. A recent analysis among 6,755 patients18 demonstrated that sirolimus-eluting stent treatment abolishes the difference in clinical outcomes at 6-month follow-up previously noted with bare-metal stents among the different lesion subsets of the modified ACC/AHA lesion morphology classification system through its positive influence on restenosis. In our study in-stent late loss for the everolimus-eluting stent among type B2/C lesions was similar compared to type A/B1 lesions rendering the above finding also applicable for the everolimus-eluting stent.
Diabetes mellitus has been repeatedly shown to be a predictor of adverse events after coronary artery revascularisations.19-22 This was also true for sirolimus-eluting stents, revealing diabetes mellitus as a negative predictor for angiographic restenosis13 and being associated with a higher late mortality after such treatment.23 Although non-significantly different, in-stent late loss was higher for both stent arms in our study among the diabetic population compared to non-diabetics. This is consistent with the higher late loss for sirolimus-eluting stents and for paclitaxel-eluting stents among diabetics compared to non-diabetics which has been previously shown.24,25
The risk of restenosis increases with lesion and stent length.26,27 Total stent length was an independent predictor for angiographic restenosis after sirolimus-eluting stents.13 In our study the long lesions had a non-significantly greater late loss in both stent arms compared to lesions < 20 mm. However the very limited number of lesions in the long lesions subgroup limits the possibility to draw any conclusion.
Interaction tests did not show any significant interactions between treatment and the analysed subgroups, except for the small vessel subgroup; meaning that the effect of the everolimus stent compared to the paclitaxel stent seems constant in the high risk subgroup compared to the remaining of the population. Except that for small vessels compared to larger vessels the in-stent late-loss seems constant for the paclitaxel arm while it is larger in the small vessels group for everolimus. However because of the limit of small sample size and impact of the outliers, further studies are needed before a solid conclusion can be drawn for this subgroup.
Limitations and conclusions
An important number of subgroups was analysed; all were not prespecified, were not controlled for during randomisation and were of relatively small sample size. All these shortcomings may directly influence these secondary results of SPIRIT II trial which may impact treatment decisions. However, the consistent difference in in-stent late loss in this study supports the superiority of the XIENCE V everolimus-eluting coronary stent in this respect compared to the TAXUS paclitaxel-eluting coronary stent among certain populations prone for higher restenosis rates.

