Abstract
Background: Drug-eluting stents have been shown to be very effective in the treatment of coronary artery disease. In this independent, single-centre registry we assess the safety and efficacy of the sirolimus-eluting stent versus bare-metal balloon expandable stent for symptomatic infrapopliteal obstructions.
Methods and results: A total of 60 consecutive patients with infrapopliteal arterial obstructions were treated by stent implantation into the tibial and peroneal arteries and the data was entered into a prospective registry. All patients were treated with balloon-expandable coronary stents with a stent length of 33 mm and a nominal diameter of 3.5 mm. Enrolment was limited to patients treatable with a single stent. 30 patients (56.7% male, mean age 71.4 years, 83.3% diabetics) received a sirolimus-eluting balloon-expandable stent. 30 patients (63.3% male, mean age 73.0 years, 76.6% diabetics) were treated with uncoated bare-metal stents. At follow-up, the cumulative rates of Major Adverse Events were 10.0% vs. 46.6%. The rates of major amputation, bypass surgery or Target Lesion Revascularisation (TLR) were all zero for the sirolimus group compared with 10.0%, 0% and 23.3% in the bare metal stent group. There were 7 deaths (sirolimus =3, bare metal =7). Angiographic follow-up comparing sirolimus vs. bare metal revealed stent occlusion 0% vs. 17.4%, restenosis >50% of 0% vs. 39.1% (p 0.0007) and mean degree of in-stent restenosis of 1.8±4.8% vs. 53±40.9% (p <0.0001) respectively.
Conclusion: In this registry sirolimus-eluting stents were shown to be safe and effective in the treatment of focal infrapopliteal obstructions.
Arterial revascularisation with restoration of straight-line, pulsatile arterial blood flow is the major therapeutic goal in patients with advanced peripheral arterial disease and chronic critical limb ischaemia, thereby achieving relief of pain or facilitating wound healing. For many patients surgical techniques are not applicable because of the general medical condition of this multi-diseased patient cohort1,2. Furthermore, the lack of suitable distal outflow vessels or the absence of venous graft material can preclude surgical bypass grafting.
Recent advances in endovascular therapy have lead to a more widespread use of interventional techniques for restoration of blood flow. Although the results of interventional therapy are promising, the occurrence of restenosis and re-occlusions may result in insufficient clinical improvement3-7. Endovascular stenting using balloon-expandable coronary stents has recently been shown to be of potential value to improve the results of infrapopliteal angioplasty for patients with critical limb ischaemia and lifestyle-limiting claudication7,8. Nevertheless, according to systematic angiographic follow-up investigations performed in our institution, re-obstruction rates of coronary bare-metal stents in tibial arteries are exceeding 50% after 12 months. Therefore, the use of drug-eluting stents could be of major benefit for the treatment of patients with tibial artery disease. The primary objective of the present study was to assess feasibility and safety of sirolimus-eluting balloon-expandable coronary stents in infrapopliteal arteries. As a secondary objective, efficacy data regarding the prevention of re-obstructions should be derived from the angiographic follow-up investigations.
Methods
Between November, 2003 and November, 2004 a total of 60 consecutive patients with infrapopliteal arterial obstructions were treated in our institution by primary stent implantation into the tibial and peroneal arteries and the data were entered into a prospective registry. The investigation was conducted in concordance with the requirements of our local ethical committee and all patients gave written informed consent prior to the procedure.
Patients with symptomatic peripheral arterial disease (Rutherford category 3 to 6) and angiographically proven infrapopliteal stenosis (>70% diameter reduction) or occlusion with a maximum lesion length of 30 mm and a reference vessel diameter of 3.0 to 3.5 mm could be enrolled. All angiographic measurements were performed by on-line quantitative angiography. In the majority of the cases patients presented with a focal obstruction of a single otherwise patent infrapopliteal artery. In 16 cases, patients with ulcerative lesions of the foot were considered for interventional treatment of an infrapopliteal obstruction in the presence of another patent tibial artery. In these cases the obstructed artery was supplying the area of the ischaemic lesion. In-flow lesions in the femoral or popliteal arteries were present in 21 cases and had to be successfully treated prior to the below-knee intervention.
In all cases 5000 units of heparin were administered during the procedure. Antiplatelet therapy was prescribed in all patients for at least 6 months as a combination of aspirin 100 mg OD and clopidogrel 75 mg OD and was continued with a monotherapy of aspirin 100 mg OD thereafter.
All patients were treated with balloon-expandable coronary stents with a stent length of 33mm and a nominal diameter of 3.5 mm. Only patients with lesions treatable with a single stent could be enrolled in this registry (Figure 1).
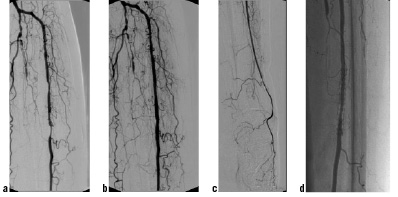
Figure 1. Single vessel supply of the left foot (posterior tibial and peroneal artery occluded) with high-grade stenosis of the anterior tibial artery (a), Good interventional result after stenting of the target lesion with a balloon-expandable bare-metal stents (Sonic 3.5/33) (b and c), Angiographic follow-up after 6 months showing mild to moderate neointimal hyperplasia within the stent (d).
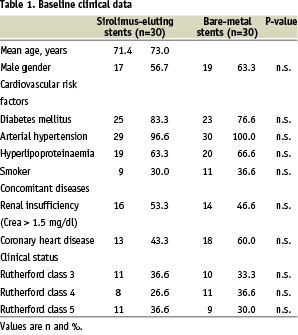
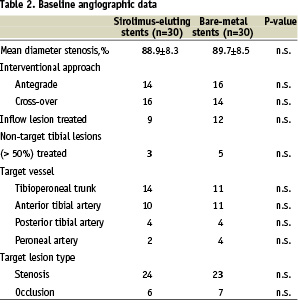
In 30 cases patients received a sirolimus-eluting balloon-expandable stent (Cypher, Cordis, Miami FL, USA). In the remaining 30 patients uncoated bare-metal stents (Bx Sonic, Cordis, Miami FL, USA) were implanted. The base-line clinical and angiographic data for both groups are given in Table 1 and 2 respectively.
Patients were scheduled in 3 months intervals for clinical follow-up visits including assessment of the clinical status and, if arteries were compressible, calculation of the ankle-brachial-index. Classification of the clinical status was made according to the Rutherford categories. Furthermore, an angiographic follow-up was performed in the time window between 6 and 12 months. Safety of the treatment was assessed for both groups by recording of deaths, amputations, need for bypass surgery and target lesion revascularisation. Efficacy parameters were primarily derived from the angiographic follow-up and included the binary restenosis rate (>50% diameter reduction) and the degree of in-stent-restenosis at follow-up. Clinical efficacy was defined as a maintained clinical improvement of at least one Rutherford category.
Statistics
Continuous variables were reported as means ± standard deviation and categorical variables as number and percentage of patients. All variables were checked concerning their Gaussian distribution with the Kolmogorov-Smirnov-test. In case of a non-Gaussian distribution values were presented with median and range.
Comparisons between the treatment groups were performed using the Chi-square-test for categorical variables. For continuous variables the paired t-test was used in case of dependency and the unpaired t-test in case of independence. A value of P<0.05 was considered significant.
Results
Acute results
Stent implantation was successfully performed in all patients without peri-procedural complications. In all cases a procedural success (<25% residual stenosis) was confirmed by angiography. There was no need for additional treatments after stent implantation (eg. additional stents). On the day after the procedure a pseudo-aneurysm at the puncture site was detected in one patient. This was subsequently treated successfully by ultrasound-guided thrombin injection.
Follow-up results
A clinical follow-up with assessment of the clinical status and recording of adverse clinical events could be conducted in all surviving patients. The mean clinical follow-up time related to the latest available clinical follow-up visit was 9.3±2.3 months in the sirolimus-group and 9.8±2.7 months in the bare-metal group (p=n.s.). During that time frame there were 7 deaths in total (3 patients from the sirolimus group and 4 patients from the bare-metal group), but none could be attributed to the interventional treatment. Additional clinical events related to the treated leg occurred only in the bare-metal group including 3 (10.0%) below-the-knee amputations and 7 cases (23.3%) of clinically driven target lesion revascularisation performed with balloon angioplasty (Table 3).
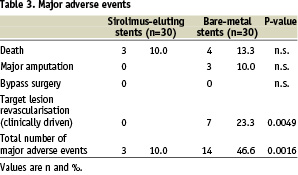
There was no need for additional stent placement. A sustained clinical improvement of at least one Rutherford category could be maintained during the follow-up period in 22 patients (81.4%) of the sirolimus group and 18 patients (69.2%) of the bare-metal group (including patients undergoing repeat interventions) (p=n.s.).
Angiographic follow-up was obtained in 24 patients of the sirolimus group and 23 patients of the bare-metal group, corresponding to 88.8% and 88.5% of the surviving patients, respectively (Table 4).
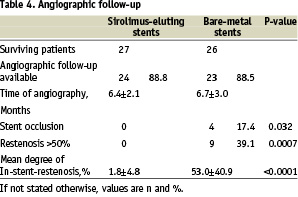
No relevant re-obstructions were seen in the sirolimus-group, whereas in the bare-metal group 9 patients (39.1%) had re-obstructions of more than 50% diameter reduction. Another 4 patients (17.4%) of the control group presented with complete stent occlusions. Similarly, the mean degree of in-stent-restenosis was substantially lower in the Sirolimus-group as compared to the bare-metal group (1.8±4.8 vs. 53.0±40.9%, p<0.0001) (Figure 2).
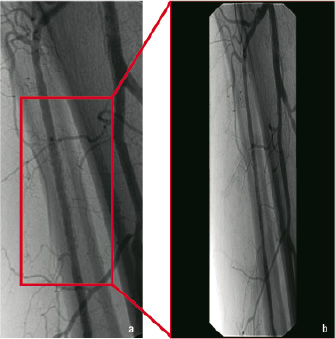
Figure 2. Typical example of angiographic follow-up performed 7 months after implantation of a sirolimus-eluting balloon-expandable stent (Cypher 3.5/33) with no evidence of neointimal hyperplasia (a, b).
Discussion
Recent advances in endovascular techniques and material have extended the applicability of interventional treatment options for patients with advanced peripheral arterial occlusive disease due to obstructions of the crural arteries. The use of endovascular stents in below knee arteries has been limited by the fact that no dedicated devices for this indication are available. Nevertheless, the results achieved with the use of balloon-expandable coronary stents are encouraging and may be superior to PTA alone7,8. Unfortunately, due to the relatively small calibre of the vessels and the diffuse nature of the disease re-obstructions are a relatively frequent finding7. According to systematic angiographic follow-up investigations performed in our institution in patients undergoing tibial stenting the re-obstruction rate after 6 months exceeds 50%.
In the coronary circulation the use of drug-eluting stents has led to a significant improvement of the follow-up results after stenting procedures. Between several available pharmacological concepts the application of sirolimus-eluting stents has been associated with consistently reduced binary restenosis rates and minimal late lumen loss as assessed during follow-up angiography9,10. Moreover, positive results have been reported for non-diabetic and diabetic patients11,12.
Based on these clinical experiences in the coronaries, the decision was made to assess the safety and efficacy of sirolimus-eluting balloon-expandable coronary stents for treatment of focal infrapopliteal lesions. In comparison to the group treated with bare-metal stents the use of sirolimus-eluting coronary stents was associated with a significant reduction of the binary angiographic restenosis rate (0% vs. 56.5%, p<0.001). Moreover, in 20 of 24 sirolimus-eluting stents with angiographic follow-up no evidence of any renarrowing could be detected, demonstrating that neointimal proliferation could be effectively prevented.
Beyond the angiographic evaluation of the binary restenosis rate, which represented the primary efficacy parameter of this investigation, clinical assessment of the patients is of major importance. In our investigation the use of sirolimus-eluting stents also resulted in a significant reduction of clinical events during follow-up. Whereas in the bare-metal group 7 patients (23.3%) required repeat interventions at the target lesion and 3 patients (10.0%) had to undergo amputation above the metatarsal level, none of these events were recorded in the group of patients treated with sirolimus-eluting stents. On first examination, this finding seems to be somehow contradictory to other reports in the literature stating that sustained patency after infrapopliteal interventions is not of major importance for a good clinical outcome and limb salvage3,14-16. In fact also in our investigation not all patients with angiographically confirmed re-obstructions experienced clinical events. On the other hand, prevention of restenosis by using sirolimus-eluting stents resulted in a significant reduction of the clinical event rate, thereby impressively showing how the utilization of drug-eluting stents could influence the clinical outcome of this highly diseased cohort of patients.
Considering the fact that there are only very limited data about the use of sirolimus-eluting balloon expandable stents in the infrapopliteal arteries, assessment of safety parameters was of major importance. In both groups a similar frequency of death was observed, which is within the range reported in other comparable investigations3,15,16. Importantly, none of the deaths were related to the peripheral arterial disease or the investigational device. Beyond that, no other clinical adverse events, including amputation, bypass surgery, and target lesion revascularisation were recorded in the group of patients treated with the sirolimus-eluting stent. In particular, no stent thrombosis was seen after treatment with the sirolimus-eluting stent.
A major area of concern related to stent treatment in peripheral arteries is the potential of stent compression or strut fracture17,18. Therefore, in our investigation the angiographic follow-ups were analysed together with the corresponding plain x-ray images demonstrating the absence of any stent compression or destruction in both study groups. Based on these findings the utilisation of sirolimus-eluting balloon-expandable coronary stents as a platform for an antiproliferative drug coating appears to be safe and feasible.
Study limitations
Most of the study limitations are related to the fact that this was an independent, non-sponsored, single-centre investigation with inherent economical and logistic restrictions. Related to this, stent usage within the study had to be limited to one stent and therefore the study cohort represents a subgroup of patients with relatively focal infrapopliteal disease. Furthermore, despite the fact that baseline clinical angiographic characteristics are very well matched, the fact that this is a non-randomised investigation limits statistical comparisons between the two patient groups. Finally every effort was made to use objective and reproducible endpoints including angiographic follow-up. However, no external core-lab analysis could be performed and therefore only a manual on-line quantitative analysis of the angiographic images could be performed.
Conclusions
According to the results of this investigation, the application of sirolimus-eluting balloon expandable coronary stents for the treatment of infrapopliteal obstructions is safe and feasible. In comparison to the results achieved in a similar patient cohort with the bare-metal version of the same stent, patients receiving the sirolimus-eluting stent showed a significantly reduced binary angiographic restenosis rate as well as a significant reduction of adverse clinical events including death, amputation and target lesion revascularisation. This treatment concept should be investigated further in larger clinical trials.