Abstract
Aims: The study sought to define the long-term angiographic and clinical outcome of a bio-engineered stent, able to sequester endothelial progenitor cells (EPC) to the stent to promote the post-stenting vascular repair response.
Methods and results: The HEALING-II was a multicentre, prospective registry, including 63 patients treated with the implantation of a Genous™ EPC capture stent. Serial quantitative coronary angiography (QCA) and intravascular ultrasound (IVUS) analysis was performed at 6 and 18 month. The 18 month composite MACE rate was 7.9%, whereas 6.3% clinically justified target lesion revascularisations were observed. Although patients received one month of clopidogrel, no (sub)acute or late angiographic stent thrombosis occurred. At 6 month follow-up, in-stent late luminal loss was 0.78±0.39 mm and percent in-stent volume obstruction was 22.9±13.7% (mean±sd). Serial angiographic and IVUS analyses were available in 30 event-free patients at post-procedure, 6 months and 18 months. From 6 months to 18 months follow-up, a significant late regression of neointimal hyperplasia was observed on QCA (late luminal loss 0.59±0.31, 24.4% reduction or 16.9% by matched serial analysis) and IVUS (percent in-stent volume obstruction 20.3±14.3%, 11.4% reduction or 9.6% by matched serial analysis). The relative increase in circulating EPC titers at long-term follow-up correlated with neointimal compaction in individual patients, suggestive of an EPC-mediated vascular repair response.
Conclusions: The HEALING II study suggests that the EPC capture stent, aimed to stimulate the coronary vascular repair response, significantly promotes late regression of neointimal hyperplasia up to 18 months after stent implantation.
Abbreviations list
EPC: Endothelial Progenitor Cell
HAMA: Human Anti-murine Antibody
HEALING: Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth
IVUS: Intravascular Ultrasound
MACE: Major Adverse Cardiac Events
MI: Myocardial Infarction
QCA: Quantitative Coronary Angiography
QCU: Quantitative intravascular coronary ultrasound
TVR: Target Vessel Revascularisation
TLR: Target Lesion Revascularisation
Introduction
Restenosis rarely occurs beyond 6 to 12 months after balloon angioplasty or bare metal stent implantation. Serial quantitative angiographic and angioscopic follow-up of bare metal stent related patients actually demonstrated regression of neointimal hyperplasia of up to 10% on QCA analysis at long term follow-up1. This neointimal compaction in bare metal-stented coronary arteries was associated on post mortem analysis with a change in the cellular and extracellular matrix composition of the neointimal lesion2. The long term restenosis regression is characterised by regeneration of the functional endothelial cell lining in the stented coronary artery, initial maturation of smooth muscle cells, and eventually the replacement of these neointimal cells by type I collagen fibres and decorin, consistent with a vascular healing response3. Drug eluting stents reduce the incidence of in-stent restenosis by way of elution of a potent cytostatic or cytotoxic compound to intervene with the inferring hyperproliferative vascular response. Concomitantly, these antiproliferative drugs seriously impede the natural healing response by delaying the formation of a functional endothelial lining over the stent or due to the chronic vascular response towards the durable stent polymer. A delayed healing response may lead to increased incidence of stent-related thrombosis, an inappropriate or impaired vasomotor response and delayed restenosis at long term follow-up (late neointimal catch-up phenomena)4,5. Histological analysis of healthy porcine and canine coronary arteries following drug eluting stent implantation indeed reveal residual fibrin deposition6 reflecting incomplete arterial healing, whereas re-endothelialisation in rabbit iliac stent implants was significantly delayed in sirolimus-and paclitaxel-eluting stents27, but not in stented healthy porcine arteries6. At long-term follow-up in animal models of stent related neointimal hyperplasia, the initial inhibition of neointimal hyperplasia following both paclitaxel and sirolimus eluting stent treatment in porcine coronary arteries was fully compensated by a delayed cellular proliferative response at 90 and 180 days. This late hyperproliferative vascular response coincided with an increase of the PCNA protein level and ongoing inflammatory response in the stented arterial segment7. Likewise, several randomised human trials have suggested a persistent and significant increase in late luminal loss and neointimal volume obstruction beyond the first 6 to 9 month follow-up in patients treated with a drug eluting stent, irrespective whether sirolimus, paclitaxel or ABT578 eluting stents were deployed8. In the HEALING II study, we sought to define the long-term benefit of bio-engineered stent specifically aimed to accelerate the vascular repair response by capture of circulating endothelial progenitor cells to the stent surface (Genous™R stent, OrbusNeich MT)10. This particular stent sequesters circulating CD34+ endothelial progenitor cells (EPC) to the luminal stent struts by immobilised anti-human CD34 antibodies, which leads to the differentiation of these captured cells into a functional endothelial layer devoid of endothelial dysfunction and capable of preventing stent-related restenosis and thrombosis. Porcine and primate studies have demonstrated the rapid and complete re-endothelialisation of the denuded arterial segments within hours following EPC capture stent placement11. The accelerated arterial repair mediated by the EPC capture technology effectively inhibited stent-related thrombus formation and neointimal hyperplasia in these baboon and pig arterial injury models. In humans, the feasibility of the EPC capture stent technology was first explored in the (HEALING-FIM) first-in-man registry in 16 CAD patients, resulting in an angiographic overall late luminal loss of 0.63±0.52 and a binary restenosis rate of 13.3%, without evidence of concomitant stent-related thrombotic events12. The HEALING II study is a multicentre, prospective, non-randomised clinical registry in 63 patients with de novo coronary artery disease to determine to determine the safety and efficacy of the bio-engineered EPC capture stent on short and long-term follow-up (6 and 18 months post PCI). The 6 month follow-up have been reported previously10. Here, we report the long-term effect of the EPC capture stent-mediated accelerated repair response on delayed restenosis (and neointimal regression) and clinical follow-up.
Materials and methods
Patient population selection
A total of 63 patients were enrolled throughout 10 medical centres in the Netherlands, Belgium and Germany. Patients were eligible for enrolment if they were between 18 and 85 years old and had a diagnosis of de novo stable or unstable angina or silent ischaemia based on a single, de novo lesion of a native coronary artery eligible for coronary stenting. The target lesion had to be less than 12 mm in length, able to be covered by a single trial stent (13 mm or 18 mm length), whereas the reference artery should measure between 2.5 and 3.5 mm vessel diameter by visual estimate. Exclusion criteria for study participation included an evolving myocardial infarction within 72 hours preceding the index procedure; renal dysfunction or suspected liver disease; unprotected left main coronary artery disease; ostial target lesions; severely calcified lesions; totally occluded vessels, excessive tortuosity of the target vessel and target lesions involving a bifurcation with a side branch that would require stenting; intervention of another lesion within 6 months before or anticipated within the scheduled follow-up of the index procedure; or left ventricular ejection fraction of less than 30 percent. The trial was reviewed and approved by the local Medical Ethics Review Committee, and written informed consent was obtained from all patients.
Endothelial progenitor cell capture stent
Endothelial Progenitor Cell Capture Stent is comprised of a stainless steel stent (9 or 18 mm R stent™, OrbusNeich Medical Technologies, Fort Lauderdale, FL, USA) coated with an adluminal polysaccharide matrix and covalently coupled monoclonal murine anti-human CD34 antibodies. These anti-CD34 antibodies specifically target the circulating endothelial cell progenitor population associated with post-natal neoangiogenesis and arterial repair response.
Study procedures
A qualifying angiogram was used to assess angiographic in-and exclusion criteria. Following verification of all angiographic and medical in-and exclusion criteria, patients signed the informed consent and were enrolled in the study. Coronary intervention was performed according to standard local institutional protocols. Pre-dilation of the target lesion was left at the investigator’s discretion, whereas post-dilation was performed as required to achieve less than 20% residual stenosis by visual assessment, with TIMI grade III flow.
Stent deployment was performed at nominal pressure as recommended, to a stent-to-vessel ratio of 1,1:1. Following stent placement, the IVUS catheter was introduced and an automated pullback was performed. The procedure was deemed angiographic successful when residual diameter stenosis within the stent was less than 20% in the presence of grade III TIMI flow, as measured by on-line QCA and the mean reference diameter of the stent was larger than 1.1 with respect to the reference diameter of the segment post-stenting (e.g. interpolated reference diameter). Procedural success was defined as an angiographic successful stent placement and the absence of cardiac death, MI, CABG or a target lesion revascularisation (TLR) event during the hospital stay. Treatment with aspirin, at a dose of 80-150 mg per day, was initiated at least 12 hours before the procedure and continued for six months post-intervention. In addition, a loading dose of 300 mg of clopidogrel was administered prior to the procedure, followed by 75 mg daily for 4 weeks. Administration of GPIIb/IIIa was left to the investigator’s discretion according to clinical practice.
Follow-up
All patients were scheduled for a clinical follow-up at 1, 6, 9 and 18 months following the implantation procedure to assess the anginal status (according to the Canadian Cardiovascular Society Classification of angina and the Braunwald Classification for unstable angina) and the documentation of MACE (including any interventional treatment [e.g.,repeat TLR] and serious adverse events). The 6 month follow-up of the HEALING II study has been reported previously10. As the 18 month follow-up was amended to the study protocol after initiation of the protocol, the long-term follow-up suffered from considerable patient drop out. Long-term clinical follow-up data was available for 39 patients (61.9%), whereas repeat angiographic follow-up data was obtained from 31 patients (49.2%) at 18 month FU. Therefore, data are presented as nonmatched, overall patient data, as well as serial analysis of patients with complete available angiographic and IVUS data sets at post-procedure, 6 months and 18 months (n=30 at 6 and 18 month FU).
Quantitative angiographic and ultrasound analysis
Coronary angiograms were obtained in perpendicular views following an intracoronary injection of nitrates. Off-line quantitative analyses of pre-procedural, post-procedural and six month follow-up angiographic data were performed at an independent imaging core laboratory (Cardialysis, Rotterdam, The Netherlands). Restenosis was defined as a reduction of at least 50 percent of the luminal diameter. Late luminal loss was defined as the difference between the minimal luminal diameter after procedure and at six months follow-up. The target lesion was defined as the stented segment plus the 5 mm segments proximal and distal to the stented segment. Intravascular ultrasound (IVUS) was performed with an automated pull back at 0.5 mm/s to examine the target lesion at post-procedure and 6-month follow-up. Lumen, stent, and external elastic membrane contours were detected with the use of CUARD QCU analysis software (CUARD BV, Wijk Bij Duurstede, The Netherlands), applying a 3-dimensional reconstruction, as described elsewhere, and submitted for off-line analysis by the independent IVUS core laboratory (Cardialysis, Rotterdam, The Netherlands). Proper stent apposition to the target vessel wall was verified by visual assessments by independent investigators of the IVUS core laboratory.
Study endpoints
The primary safety endpoint of the study was defined as a composite of MACE at 30 days defined as the incidence of cardiac death, Q-wave or non-Q-wave myocardial infarction, emergent cardiac surgery and clinically justified target lesion revascularisation (TLR) within 30 days following the index procedure. A TLR/TVR was considered clinically justified when the diameter stenosis was more than 50% (by off-line QCA) and either, (1) a history of recurrent angina pectoris presumably related to the target vessel, or (2) ischaemia-related ECG changes at rest or during exercise test related to the target vessel or either (3) an abnormal result of any invasive functional diagnostic test (including Doppler flow velocity reserve or fractional flow reserve). A TLR/TVR with a diameter stenosis ≥70% (by off-line QCA) in the absence of the afore mentioned ischaemic signs or symptoms was also considered clinically driven. The primary efficacy endpoint was percent in-stent volume obstruction at 6 months as assessed by off-line IVUS. Secondary safety endpoints comprised MACE rate and device-related SAEs until 18 months follow-up and the incidence of stent thrombosis as documented by coronary angiography. An independent Clinical Events Committee adjudicated all major adverse cardiac events. A data safety monitoring board reviewed safety data on a regular basis. Secondary efficacy endpoints included quantitative coronary angiography-derived vessel parameters of the in-stent segment and 5 mm proximal and 5 mm distal from the edge of the stent: including acute gain, MLD, mean diameter, % diameter stenosis and binary restenosis rate, angiographic and procedural success, clinically justified TLR free rate at 18 months, quantitative IVUS assessment, including proper stent apposition. Stent thrombosis was defined as an angiographically documented complete occlusion or a flow limiting thrombus (TIMI flow 1 or 2) of a previously successfully treated artery.
Adverse events
The Data Safety Monitoring Board (DSMB) was responsible for the review of data and identification of any potential safety issues. Members of this board were not affiliated with Orbus MT, Cardialysis, or investigators and had to declare any conflicts of interest should these arise. The Clinical Event Committee, consisting of 3 independent physicians convened at various intervals during the study to confirm the classification of the following endpoints: all deaths; myocardial infarction and target vessel and target lesion revascularisation by percutaneous intervention or bypass surgery.
Analysis of circulating CD34+/KDR+ endothelial progenitor cells by fluorescence aided flow cytometry
The efficacy of the EPC stent to accelerate arterial repair and prevent coronary neointimal hyperplasia could be affected by the titer and functional properties of the endogenous circulating EPCs. To quantify the circulating endothelial progenitor cells (EPCs) by quantitative flow cytometric analysis in the patients, at 6 months and 18 months follow-up, whole blood was incubated with 7AAD viability dye, APC-labelled anti-hCD45, FITC labelled anti-hCD34 (Pharmingen) and PE-labelled anti-hKDR (R&D systems) according to the manufacturer protocol (and ISHAGE guidelines) for 20 minutes at room temperature using Stem-Kit™ Reagents; Beckman Coulter Comp, Cedex, France). After addition of counting fluorospheres (Beckman Coulter, Cedex, France), the red cell fraction was lysed by NH4Cl and analysed on an automated flow cytometer (FACSCanto®, Becton&Dickenson). Corresponding isotypic IgG1 controls were used to set proper gating for each antibody. Whole blood samples were analysed by flow cytometry within 24 hours after collection at the different sites. Endothelial progenitor cell (EPC) titer was expressed as absolute number of viable EPCs per 100 µl of whole blood (7AAD–/CD45+/CD34+/KDR+). EPC levels were post hoc cross correlated with the clinical, angiographic and IVUS outcome of patients enrolled in the HEALING II registry.
Statistical analysis
The HEALING II study was designed as an exploratory, prospective, non-randomised trial. No prior formal power calculations were conducted to determine the efficacy of the EPC capture stent technology. The primary analysis was performed according to the intention to treat principle and included all patients initially enrolled in the study. Continuous various variables are summarised by mean±standard deviation. Post procedural and 6 month and 18 months follow-up angiographic and IVUS changes were compared using a 2-tailed paired t-test. P<0.05 was considered to be statistically significant. Only events adjudicated by the Clinical Event Committee have been taken into account for the analysis of MACE.
Results
Baseline characteristics and procedural outcome
Overall, 63 patients were included in the HEALING-II registry between May and October 2004. Table 1 and 3 present the detailed baseline characteristics and procedural outcomes for this patient population.
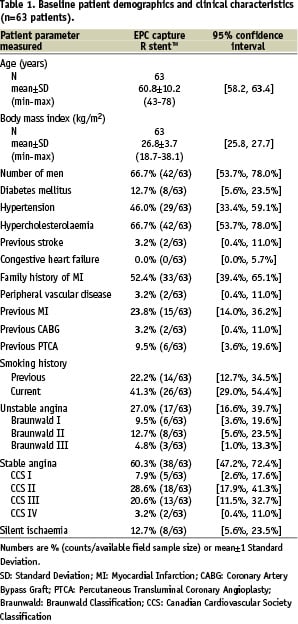

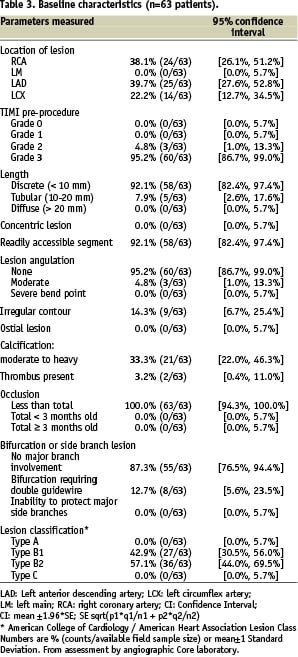
Baseline demographics and clinical characteristics showed that patients had a mean age of 61 years, 66.7% male participation and 12.7% suffered from diabetes mellitus. Overall angiographic and procedural success were both 93.7% (59/63). In one patient the delivery system could not cross the target lesion and eventually a conventional bare metal stent was implanted. A second overlapping EPC capture stent was implanted in four patients to treat a dissection following implantation of the first stent, and in one patient due to incomplete coverage of the target lesion by the first stent.
Clinical outcome
Compliance to clinical follow-up at 6, 9 and 18 months follow-up was respectively 98.4%, 87.3% and 61.9%. Table 2 provides an overview of the MACE events at 1, 9 and 18 months. One patient died at day 90 post-implantation, after falling down the stairs at home. As the event was neither witnessed nor was permission for autopsy acknowledged, cardiac death could not be excluded. Therefore, this patient was adjudicated as cardiac death. At 1 month clinical follow-up, this patient has been event and angina-free. Another patient died at day 270 days following stenting after being diagnosed with ostial and pulmonary metastasised synovial sarcoma, 3 months following the index procedure. The patient was adjudicated by the CEC as a non-cardiac death, not associated with the investigational study device. No acute or subacute thrombosis occurred in patients over the 18 month time period despite one month of dual antiplatelet therapy. Myocardial infarction did not occur in any of the patients during the 18 month follow-up. At 18 month follow-up, target lesion revascularisation (TLR) by repeated PCI occurred in 11 out of 63 patients (17.4% see Table 2), of which 4 were adjudicated clinically-driven (6.3%). Revascularisation of these events was based on ischemic symptoms (3/4), a positive stress test (1/4), and ischaemic ECG changes (1/4). An angiographic stenosis with a diameter stenosis of more than 70% on QCA (angiographic indication for TLR event) did not occur in any of the patients. Target vessel revascularisation (TVR) by repeated PCI occurred in 5 out of 63 patients (7.9%). Total adjudicated MACE rate (including cardiac death, myocardial infarction, CABG, clinically-driven TLR) of the EPC capture stented patients was 7.9% (5/63). All clinically driven and non-clinically driven MACE events (including all death, myocardial infarction, CABG, total TLR and TVR) was 30.2% (Table 2).
Six and eighteen month angiographic and IVUS analysis of target vessel
Compliance to angiographic follow-up at 6 and 18 months follow-up was respectively was 92.1 and 49.2% (58/63 and 31/63 patients). At 6 months follow-up, in-stent MLD was 1.67±0.51 mm (post procedural in-stent MLD 2.45±0.35), whereas late luminal loss was 0.78±0.39 mm on QCA (mean±SD; Table 4).

The in-stent binary restenosis rate at 6 month angiographic follow-up was 17.2% (10/58 patients), whereas the overall diameter in-stent stenosis by QCA was 34.91±13.63%. At 18 month follow-up, angiographic late luminal loss regressed significantly in the EPC-capture stented arteries to 0.59±0.31 mm (Figure 1A: 24.4% reduction as compared to 6 month FU; 16.9% reduction using matched serial data).
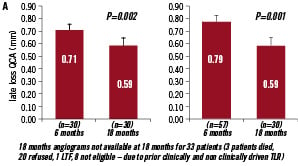
Figure 1A. Late luminal loss (in mm) as measured by QCA at 6 and 18 month follow up. Matched serial follow-up of late luminal loss by QCA analysis (left) at 6 months follow-up (0.71±0.35 mm; mean±SD) and at 18 month follow-up (0.59±0.31 mm; 16.9% reduction as compared to 6 month FU); QCA derived late luminal loss of all patients enrolled (right) at 6 months (0.78±0.39 mm; mean±SD) and at 18 month follow-up (0.59±0.31 mm; 24.4% reduction as compared to 6 month FU).
Percent diameter stenosis was reduced to 29.65±11.74%. IVUS analysis did not reveal malapposition of the stent in any of the study patients. Concurrent with the angiographic data, quantitative ultrasound data also demonstrated late neointimal regression in the stented arterial segments, although less pronounced as suggested by QCA. In-stent volume obstruction as assessed by IVUS was respectively 22.9±13.7% and 20.3±14.3% at 6 and 18 month FU (11.6% reduction as compared to 6 month FU; plaque volume respectively 31.3±18.8 mm3 and 27.4±19.4 mm3; plaque behind stent volume 150.5±62.0 mm3 and 143.9±64.3 mm3).
Flow cytometric analysis of EPC titer
Quantitative flow cytometric analysis was used to determine circulating EPC levels and cross correlated with angiographic outcome. At the 18 months follow-up, EPC titers were analysed in 29 out of 31 patients and correlated with angiographic outcome (Figure 1B).
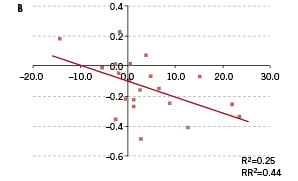
Figure 1B. Flow cytometric analysis of endothelial progenitor cell levels in study patients. Change of endothelial progenitor cell titer (absolute number of EPC/ 100 µl whole blood) cross-correlated with change in angiographic late luminal loss. Compaction of late luminal loss correlated with an increase in EPC level, suggestive of an EPC mediated vascular healing response underlying late restenosis regression at 18 month follow-up.
EPC data analysis of the 6 month clinical follow-up was unavailable in two patients. Absolute change in EPC levels between 6 and 18 month follow-up correlated with late regression in QCA late luminal loss (RR2=0.44; P=ns; Figure 1B). This would suggest that the process of late neointimal regression is, at least in part, induced by an EPC-mediated vascular repair response of the injured arterial segment. Likewise, absolute EPC titers correlated with 18 month angiographic outcome, although this association was less apparent as compared to the earlier reported 6 month time point.
Discussion
Main findings
The HEALING II was a prospective multicentre clinical study to assess the safety and efficacy of stent strategy aimed to accelerate the endogenous vascular repair response in the injured arterial segment following balloon angioplasty and stenting. Circulating endothelial progenitor cells (EPC) are captured by a stent coating containing antibodies directed against the CD34 EPC marker. In large animal models, the sequestered EPCs to the stent surface rapidly differentiated into a functional endothelial cell lining within hours following stent placement, which initiates the vascular repair response, and eventually re-establishes vascular homoeostasis by promotion of cellular senescence of vascular cell components, prevention of platelet aggregation and reinstatement of normal vasomotor function13. In various large animal studies in pigs and primates, implantation of the EPC capture stent resulted in complete re-endothelialisation of the stented segment within 48 hours, inhibition of neointimal formation, reduced thrombogenicity and sustained vasomotor function under phenylephrine stimulation, reflecting accelerated vascular repair, resulting in long-term preserved endothelial function14. Previously, we have reported the 6 month angiographic and IVUS follow-up data of the HEALING II clinical registry10. At 6 month follow-up, angiographic and ultrasound outcome of the EPC capturing technology correlated closely with the circulating EPC titer. Patients with a normal EPC level responded favourably to the bio-engineered stent with a late luminal loss of 0.53±0.06 (mean±sd) and percent neointimal volume obstruction of 14.8±2.87%. In contrast, low EPC levels were associated with a late luminal loss and % volume obstruction comparable to uncoated bare metal stenting (late luminal loss: 1.01±0.07;% volume obstruction: 28.8±2.87%), and TLR/TVR events. Patients were invited to an 18 month angiographic and clinical follow-up to evaluate an effect of accelerated vascular repair on long term neointimal regression. Patient compliance to this study amendment for clinical follow-up was 61.9% (39 patients). Although not powered to detect significant changes in thrombogenicity, no in-stent thrombosis was detected in the study group up to 18 month of observation, despite the limited time of the dual antiplatelet therapy. Also, no adjudicated MACE event has been reported during the period between 9 and 18 month follow-up, resulting in an overall 7.9% MACE rate over the 18 month study period. Remarkably, neointimal hyperplasia regressed significantly between 6 and 18 month follow-up, from 0.78±0.39 to 0.59±0.31 mm at 18 month follow-up (–24.4%, or –16.9% by matched serial analysis). Neointimal volume, as assessed by quantitative IVUS analysis, likewise regressed, although less pronounced (% volume obstruction 22.9±13.7% [6 month FU] to 20.3±14.3% [18 month FU], 11.4% reduction or 9.6% by matched serial analysis). We postulate that the late restenosis regression beyond the 6 month angiographic follow-up is, at least, in part mediated by an ongoing vascular repair response accelerated by the EPC capture stent technology.
Various animal and human studies have shown mobilisation of circulating EPCs and CD34+cells with endothelial damage, vascular trauma and acute myocardial infarction, reflecting increased endothelial cell turnover and ongoing vascular repair13. Stimulation of EPC levels in various animal models by exercise15, EPC infusion16 or either pharmacotherapy with GCSF17, oestrogen18, statin19 or erythropoietin20, resulted in accelerated re-endothelialisation and reduced neointima formation. In CAD patients, EPC cell count were shown to correlate closely with preservation of flow-mediated brachial artery reactivity irrespective of other conventional cardiovascular risk factors or Framingham risk score, indicative that circulating EPCs convey endothelial integrity and function, and EPC titers predict endothelial function in atherosclerotic vascular disease13. Moreover, EPC levels were a significant independent predictor of MACE events, including death from cardiovascular causes, incidence of first major cardiovascular events, revascularisation or hospitalisation, in 519 CAD patients during a one year follow-up3. These studies substantiate the hypothesis that EPCs function as a cellular reservoir, that contribute to ongoing arterial repair, whereas continuous endothelial injury or dysfunction by cardiovascular risk factors leads to an eventual exhaustion or depletion of a presumed finite supply of EPCs, permitting the perpetual progression into atherosclerotic disease21. Accelerated arterial repair through sequestration of EPCs would have multiple beneficial effects, including rapid reinstatement of the endothelial function and inhibition of neointimal formation, in addition to, inhibition of platelet aggregation and thus, reduced thrombogenicity of the stented vessel, improved vasomotor function, and ultimately attenuation of ongoing atherosclerosis progression.
On long term, accelerated vascular healing may reinstate proper endothelial function and potentiate late neointimal regression. On post mortem examination, this neointimal regression has been also been related to a vascular healing response with regeneration of the endothelial lining, maturation of the neointimal smooth muscle cells, and change in the composition of the extracellular matrix by which proteoglycans were replaced by type I collagen fibres and decorin23. Neointimal regression was previously described between 6 months and 4.5 years following balloon angioplasty24. In bare metal stent-treated patients, neointimal compaction occurred beyond 6 months and 3 years follow-up as shown by angiographic increase of MLD by 10%25. Asakura confirmed and extended these observations by angioscopic analysis in a smaller group of patients26 suggestive of late neointimal regression and ongoing vascular healing. Consistent with these observations, the EPC capture stent technology resulted in our study in a significant regression of neointimal hyperplasia by 24.4% with an angiographic late luminal loss of 0.59 at 18 month follow-up.
In contrast, drug eluting stents have shown a significant and persistent progression of neointimal hyperplasia by IVUS and QCA analysis on long term follow-up, regardless of the use of paclitaxel, ABT578 or sirolimus. The issue of a “late catch-up phenomenon” (delayed restenosis) which was first observed after coronary brachytherapy,28 has not been fully investigated with DES. The cause of late restenosis related to DES may be multifactorial and may be attributed to a delayed vascular healing in combination with an ongoing response to the bioactive pharmaca or stent polymers. At post mortem analysis of 40 cases previously treated with DES, both paclitaxel and sirolimus eluting stents were indeed associated with a delayed healing response, characterised by persistent fibrin deposition, incomplete reendothelialisation and evidence of (mural) late stent thrombosis, as compared to matched autopsies of BMS implantation2. The notion of incomplete coverage of drug eluting stent at long-term follow-up associated with increased incidence of non-occlusive mural in stent thrombosis was also corroborated in angioscopy studies of DES-stented patients28. These observations underscore the importance of the natural vascular healing response following arterial (balloon) injury, and stress the need for concomitant stent-related approaches to compensate the incomplete vascular healing in DES-stented coronary arteries.
A stent specifically aimed to capture circulating EPCs, promotes the endogenous vascular repair response in the injured arterial artery, and thereby not only prevents neointimal hyperplasia and endothelial vasomotor function, but also may also potentiate neointimal regression on long term follow-up related to this natural healing response. The magnitude of restenosis compaction after 6 month follow-up, was related to the change in EPC titer between 6 and 18 month follow-up suggestive that neointimal compaction was indeed related to an EPC mediated repair response.
Previously we reported that in patients with normal levels of EPCs, treatment with the EPC capture stent resulted in a reduction of restenosis formation after 6 month follow-up. Moreover, between 6 months and 18 month FU, regression of angiographic late luminal loss appeared to be facilitated by the accelerated and ongoing vascular healing induced by the EPC capture stent. Further study and development of this promising technology is needed to confirm the clinical efficacy of this bio-engineered stent. Due to the small sample size and the exploratory nature of the present study, it did not fully evaluate the long-term efficacy of this device. To substantiate and extend the findings in the HEALING FIM and the current HEALING II clinical studies, a forthcoming HEALING IIB clinical study will assess the safety and short-and long-term effectiveness of the Genous™ Bio-engineered R stent, in conjunction with optimal statin therapy, in the treatment of elective patients with de novo native coronary artery lesions.
Acknowledgements
Funding Sources:
Study supported by OrbusNeich Medical Technologies, Florida, USA
Independent Imaging Core lab (QCA/QCU) and data monitoring by Cardialysis bv, Rotterdam, The Netherlands.

