Abstract
Transcatheter mitral edge-to-edge repair (TEER) is an established procedure for managing mitral regurgitation (MR) in high-risk patients. It is effective in treating both primary and secondary MR, as reported in the surgical and interventional literature. Over time, TEER has gained popularity and achieved procedural success in various anatomies. The less invasive nature of TEER, along with its high safety profile and immediate haemodynamic improvement suggest potential benefits in high-risk populations who are not normally included in major trials. These patients, often deemed unsuitable for surgical intervention, are typically managed conservatively, despite accumulating evidence suggesting the potential of clinical improvement by reducing MR through TEER. Examples include post-myocardial infarction MR, patients with hypertrophic obstructive cardiomyopathy and patients experiencing recurrent MR after surgical intervention. This review discusses the utilisation of TEER beyond recognised indications, examining outcomes and limitations in diverse patient populations. Further studies are warranted to evaluate the benefits of TEER in clinical scenarios beyond the current indications.
Mitral regurgitation (MR) is the second most frequent valvular heart disease in Europe1. The underlying mechanism (primary or secondary) determines the therapeutic approach, and predicts procedural complexity and outcomes. Interest in mitral valve repair was regained in the late eighties, and exactly 40 years ago, a seminal publication, the “French Correction," established fundamental rules and a systematic approach to valve repair2. However, repair was limited to patients receiving primary repair of the central portion of the posterior leaflet, while anterior and bileaflet prolapse were considered complex anatomies, often managed with valve replacement.
In the nineties, Ottavio Alfieri developed the edge-to-edge repair technique, a revolutionary approach to restore mitral valve function in complex anatomical scenarios. The technique, restoring coaptation at leaflet level, was shown to be effective regardless of the underlying mechanism of regurgitation. As a consequence, the edge-to-edge technique soon demonstrated its efficacy in diverse pathologies, becoming the most versatile repair method for MR3. Its simplicity also allowed the development of transcatheter mitral edge-to-edge repair (TEER) − the most successful percutaneous approach for MR to date.
Current guidelines suggest that TEER is a safe alternative for patients with severe MR who have surgical contraindications or are at a high operative risk4. Several registries and 2 randomised trials have evaluated the impact of TEER in patients with severe secondary MR (SMR) compared with guideline-directed medical therapy: MITRA-FR (Multicentre Randomized Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients With Severe Secondary Mitral Regurgitation)5 and COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation)6. The MITRA-FR Study showed no improvement in outcomes, while the data from the COAPT Trial showed a mortality benefit for TEER. In light of the conflicting data, subsequent guidelines recommended TEER in selected patients with severe SMR fulfilling the COAPT inclusion criteria4. Due to its less invasive nature, high safety profile, and frequently instantaneous haemodynamic improvement, TEER is an attractive technique among high-risk populations that do not fulfil the COAPT criteria, including patients who require urgent mitral intervention, patients with complex mitral anatomies, and patients with advanced heart failure (HF), cardiomyopathies or congenital heart disease (CHD). It is important to recognise the diversity among these high-risk populations and to understand that the level of supporting evidence for TEER may differ based on the specific underlying causes or aetiologies of MR. For instance, the data substantiating TEER in cases with complex anatomy are more robust compared to the more restricted evidence available for TEER in patients with CHD, which mainly relies on case series. This review discusses several of these patient populations with the available data to support the utilisation of TEER in each population (Central illustration).
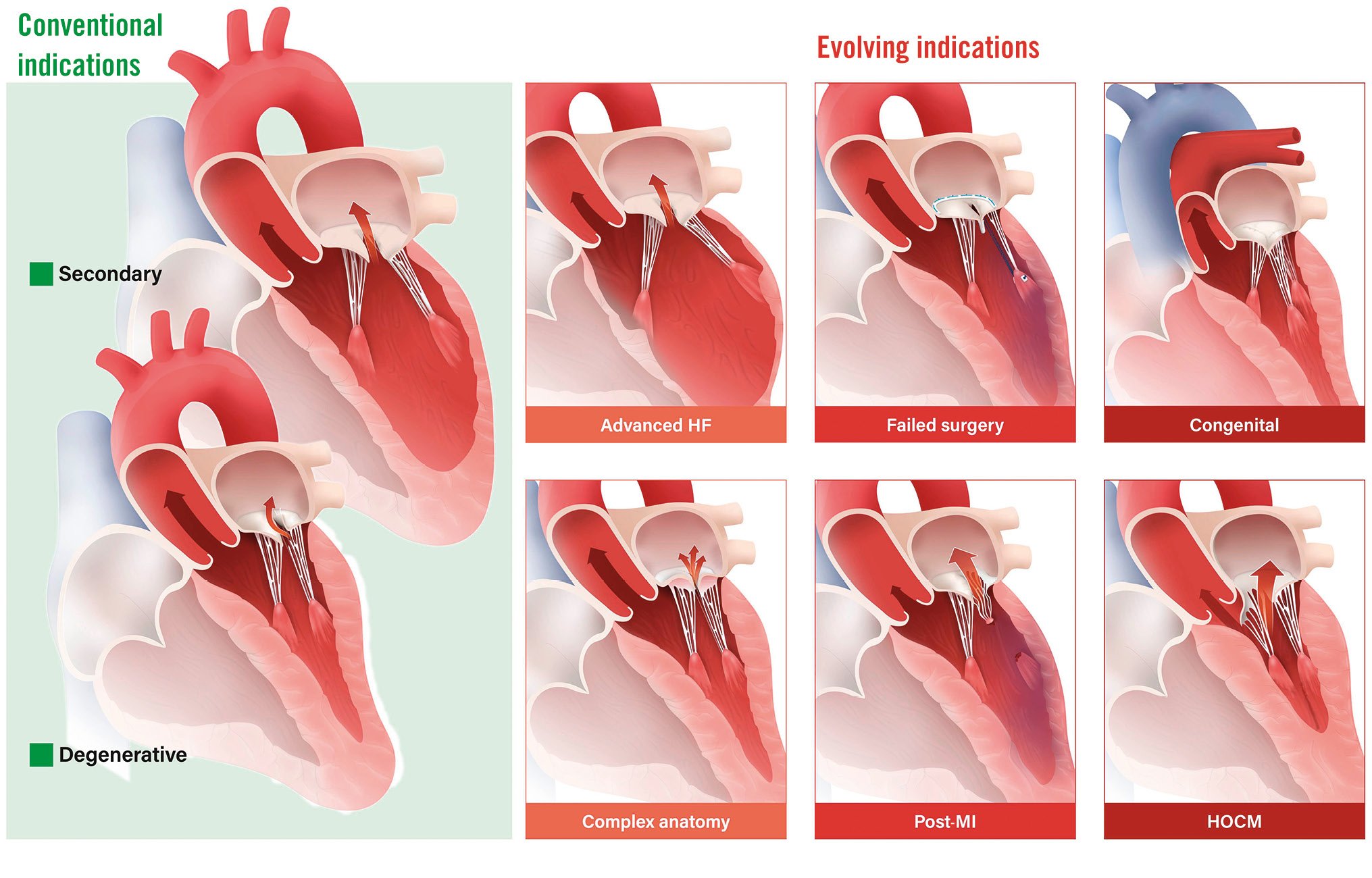
Central illustration. Clinical indications for mitral TEER. Conventional and evolving indications for TEER, according to the available supportive data, from the most established (advanced HF and complex anatomy, depicted in orange) to the more limited evidence (HOCM and congenital heart disease, represented in dark red). HF: heart failure; HOCM: hypertrophic obstructive cardiomyopathy; MI: myocardial infarction; TEER: transcatheter edge-to-edge repair
Urgent TEER for acute MR
Acute mitral regurgitation (AMR) is a serious medical condition that requires urgent treatment. Common aetiologies for AMR include the following: post-myocardial infarction (MI), primary or secondary MR, and acute chord rupture in patients with degenerative mitral valve disease. Patients with HF due to or worsened by acute decompensated MR are typically high risk for surgical intervention and were excluded by previous TEER trials, including the COAPT Trial. The goal of medical management is to stabilise the patient and relieve symptoms. This may include intravenous diuretics, vasodilators, inotropic support, and potentially mechanical support78. Surgical intervention is considered for patients who continue to experience refractory symptoms despite receiving the best medical care, but high-risk patients who are deemed inoperable frequently experience unfavourable results9. In these situations, surgical mitral valve replacement (SMVR) often carries a very high risk, especially when compared to elective procedures, with a 30-day mortality rate of 22.5%, ranging from 14.8% for patients with degenerative valve disease to 26.9% for patients with recent acute MI10. The variation in clinical presentations of AMR along with the pitfalls in diagnosis have led to non-uniform management of AMR and divergent outcomes, particularly in patients considered high risk for surgical intervention. An important group of critically ill patients are those with AMR due to a recent MI. Primary MR due to papillary muscle rupture or rapid remodelling of the infarcted left ventricle (LV), resulting in apical and inferior displacement of the papillary muscles, can cause post-MI SMR (Figure 1). The acute onset of regurgitant volume overload to a non-compliant left atrium (LA) causes a rapid increase in LA pressure, increased pulmonary pressures, pulmonary congestion, and subsequent LV failure. According to the available data, TEER has been associated with clinical improvement in the majority of patients with AMR1112, including those with severe LV dysfunction13. The procedure, despite in some cases being technically demanding, has also been reported to have encouraging clinical outcomes even in patients with cardiogenic shock, as long as initial haemodynamic stabilisation was achieved prior to TEER14. Recently, the International Registry of MitraClip in acute mitral regurgitation following acute myocardial infarction (IREMMI) published results on 471 patients with post-MI SMR, collected from 21 centres. Patients had at least moderate to severe MR and HF symptoms within 90 days of MI and were managed using SMVR, TEER, or without intervention (conservative group). Surgical intervention was carried out sooner than TEER (median of 12 days from the date of MI [interquartile range {IQR} 5-19] vs 19 days [IQR 10-40]; p=0.01). Although the immediate procedural success rates for surgery and TEER were comparable, the in-hospital and 1-year mortality rates in the surgical group were significantly higher, suggesting that TEER may be a superior alternative to surgery in high-risk patients with significant post-MI AMR15. Recently, an analysis of TEER procedures performed in patients with cardiogenic shock from the Society of Thoracic Surgeons/American College of Cardiology (STS/ACC) Transcatheter Valve Therapy (TVT) Registry showed that 30-day mortality in patients having undergone a successful procedure was lower than the STS expected rate. At 1 year, mortality was significantly lower in patients who had undergone a successful TEER procedure, compared to those in whom this procedure had failed (29.6% vs 45.2%, 95% confidence interval: 0.42-0.62)16.
However, these observational data should be considered hypothesis-generating, and the outcomes may be confounded by unmeasured variables, including that patient allocation to each therapy may have been biased by clinical factors which affected physician decision-making. The ongoing MINOS trial is randomising participants in cardiogenic shock and with at least moderate MR to receive either TEER or medical therapy, which will probably shed more light on this population17.
In addition, it is important to note that TEER is a relatively new and still-evolving procedure, and its long-term efficacy and safety are not yet fully established. Furthermore, the fibrosis that occurs after TEER on the valve leaflets renders an eventual surgical repair nearly impossible. Although this problem has a lesser effect on patients with SMR who are most often treated with a replacement valve, the acute treatment of a potentially repairable valve with primary MR is a matter of concern in the case of TEER failure. Furthermore, not all patients with AMR may be candidates for TEER, and other treatment options, such as surgical repair or replacement of the mitral valve, may be necessary in some cases.
In summary, TEER appears to be a promising salvage procedure (even as a bridge to SMVR), particularly in critically ill patients with AMR due to a recent MI.
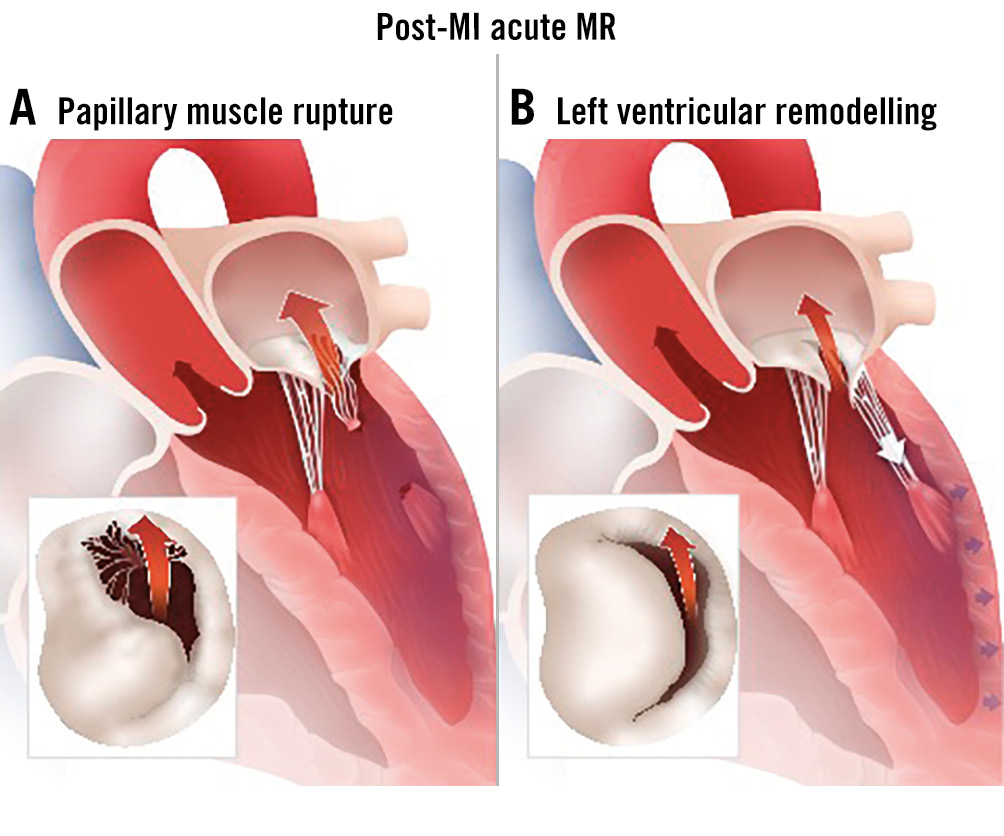
Figure 1. Post-MI acute MR. A) Papillary muscle rupture and B) rapid LV adverse remodelling causing SMR. LV: left ventricular; MI: myocardial infarction; MR: mitral regurgitation; SMR: secondary mitral regurgitation.
TEER in patients with severe LV dysfunction or advanced HF
Patients with severely reduced LV dysfunction and severe MR typically face poor outcomes. These patients share similar features to those included in the MITRA-FR trial; they are often debilitated by symptoms, and medical therapy is limited because of their comorbidities. For example, patients with low blood pressure and kidney dysfunction may not tolerate guideline-directed medical therapy, as this might further decrease blood pressure. The role of TEER in these patients has not been determined, and data on these patients were mostly obtained from registries. The multicentre German transcatheter mitral valve interventions (TRAMI) registry compared the data of 256 patients with severely reduced LV function (ejection fraction [EF] <30%). These patients had a more advanced stage of disease with a more pronounced elevation of N-terminal pro-brain natriuretic peptide (NT-proBNP) levels. The clinical benefit of patients with severely reduced LV function undergoing TEER with the MitraClip device (Abbott) was significant, persisted during the 1-year follow-up period, and was comparable to the benefits in patients with better LV function18.
A recent study evaluated 118 subjects with New York Heart Association (NYHA) Class IV at baseline who were treated with MitraClip and subsequently showed a marked improvement in NYHA Functional Class and Kansas City Cardiomyopathy Questionnaire (KCCQ) score at 1 year. A total of 72.6% of them were of NYHA Class I/II at 1 year (p<0.0001, compared with baseline). Interestingly, NYHA Class IV subjects had a numerically higher score for quality-of-life improvement than those in Class III or lower (+31.2 vs +20.7) at 1 year, which can potentially be attributed to the lower KCCQ score at baseline. However, 1-year mortality and HF hospitalisation rates remained high in NYHA Class IV subjects19.
Analysis of 119 patients from the MitraBridge registry with severe SMR and advanced HF (median EF: 26%) were treated with MitraClip as a bridge strategy to heart transplantation. The procedure was safe, and two-thirds of patients remained free of adverse events at 1 year20. In the MITRA-FR trial, the addition of MitraClip to medical therapy did not lead to a significant reduction in death or HF hospitalisations compared to medical therapy alone5. In contrast, the COAPT Trial demonstrated that TEER with MitraClip in addition to medical therapy reduced HF hospitalisations and improved survival compared to medical therapy alone6. One explanation for the disparity was that patients with significantly depressed LV function, such as those in the MITRA-FR trial, may not benefit from TEER21. However, a subanalysis of the COAPT Trial suggested that all subclasses of patients, including those with significantly impaired LV function (EF ≤30%), benefit from TEER22. On the other hand, the opposite is also true: MITRA-FR could not identify any subset benefitting from the MitraClip.
Afterload mismatch is an important concern for patients with low EF and SMR undergoing mitral valve interventions. It occurs when MR abruptly disappears after the procedure, leading to acute haemodynamic instability and low cardiac output due to the compromised ability of the LV to handle increased afterload. A recent study showed that about 20% of patients with MR experience a significant drop in their EF (≥15%) after mitral TEER, indicating afterload mismatch23. Predictors of this include a low baseline EF, an elevated left atrial pressure (V waves >30 mmHg), and pre-existing right ventricular dysfunction2324. The condition is typically reversible as the LV adapts over time. Close monitoring in the postprocedural period is essential. Inotropic therapy may be needed to support the heart and improve cardiac output in severe cases24. Identifying at-risk patients and vigilant post-procedure monitoring are crucial for successful management.
Importantly, both the elderly population (with comorbidities) and the younger population (with depressed LV function) are included in the heterogeneous advanced HF group. To create the best treatment plan, clinicians must carefully balance the risks and advantages of each therapy, including mechanical support and heart transplantation. Despite better results from new mechanical circulatory support technologies, managing this population is challenging, because many patients are not good candidates, while others frequently require bridging therapies to slow the progression and worsening of HF.
TEER in systolic anterior motion-related MR and hypertrophic cardiomyopathy
In hypertrophic obstructive cardiomyopathy (HOCM), beyond hypertrophy, an important pathophysiological feature is systolic anterior motion (SAM) of the mitral valve leaflets, frequently leading to a combination of dynamic left ventricular outflow tract (LVOT) obstruction and MR25.
The cornerstone of care is pharmaceutical therapy, followed by septal reduction therapy and/or mitral valve surgical intervention2627. Alcohol septal ablation may be beneficial for patients who are at high surgical risk28. However, septal ablation may not be possible as an alternative therapy because of anatomical issues, such as when the septum is insufficiently broad. In these cases, TEER may be a viable strategy to manage the LVOT obstruction while also relieving the MR by reducing the SAM (Figure 2). Sorajja et al presented 5 treated patients in whom a MitraClip procedure was successful in reducing the intraoperative LVOT gradient, LA pressure, and MR grade. The procedure was also associated with an improvement in cardiac output in a subset of patients. Additionally, all patients experienced symptom improvement and were able to achieve NYHA Functional Class I or II over the course of the follow-up period29. The data on TEER in HOCM are still anecdotal and based on small case series; therefore, additional studies are required to validate these preliminary data. However, symptomatic patients with prominent SAM-related MR and LVOT obstruction who are not candidates for alcohol septal ablation30 or deemed high risk for surgical intervention should be considered for TEER.
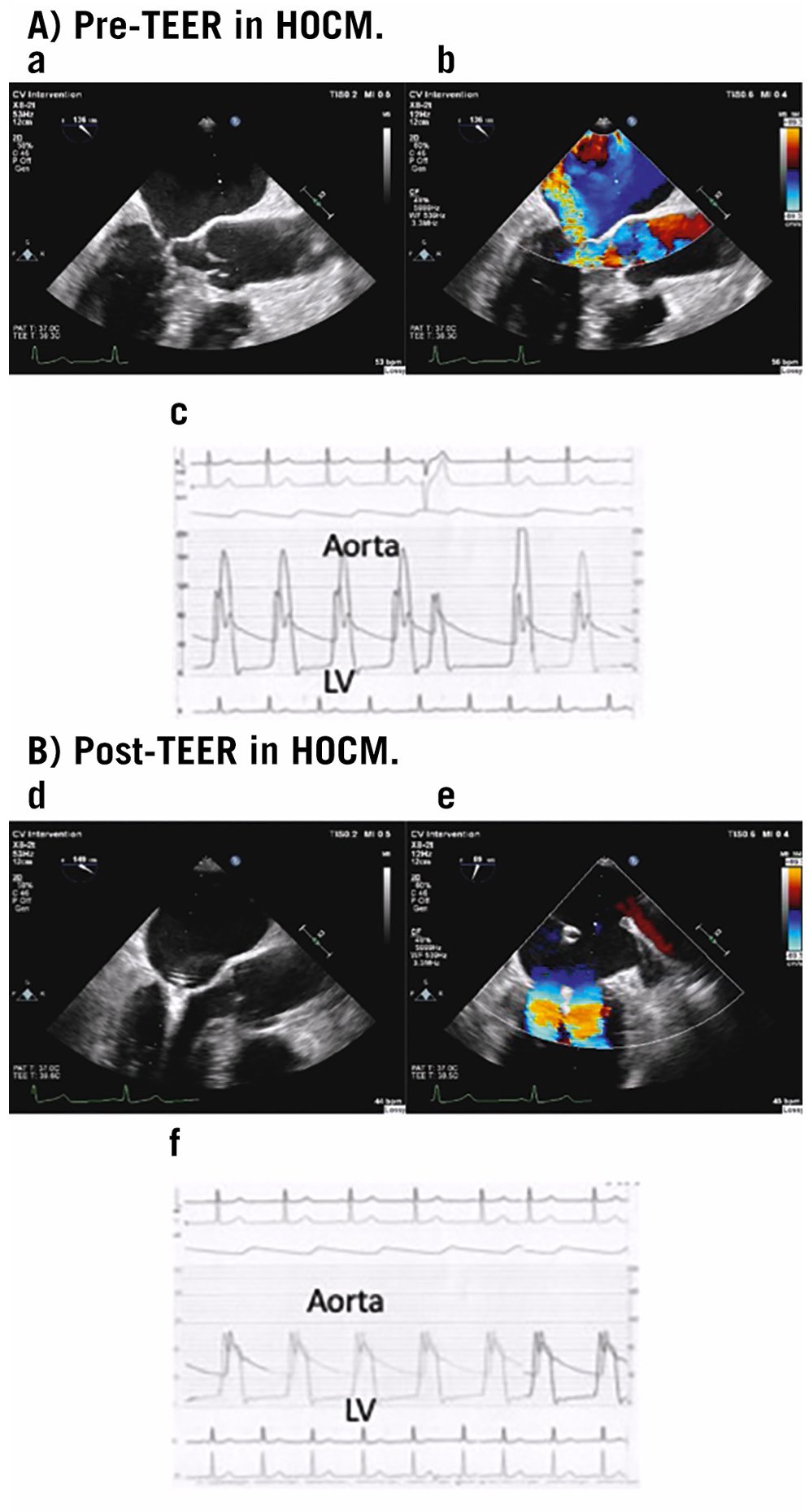
Figure 2. Pre-TEER and Post-TEER in HOCM. A) Pre-TEER in HOCM. Severe SAM-related MR (a,b) and simultaneous tracing of the aorta and LV pressure showing LVOT gradient at rest as 139 mmHg (c). B) Post-TEER in HOCM. Following MitraClip single XTR device implantation, preventing SAM eliminated the MR (d,e) and simultaneous tracing of the aorta and LV pressures shows the obliteration of the LVOT obstruction (f).HOCM: hypertrophic obstructive cardiomyopathy; LV: left ventricular; LVOT: left ventricular outflow tract; MR: mitral regurgitation; SAM: systolic anterior motion; TEER: transcatheter edge-to-edge repair.
TEER after a failed surgical intervention
Patients who experience recurrent MR following surgical mitral intervention are often at high risk for additional surgical intervention. Redo-mitral valve surgery can be technically difficult; it is linked to non-negligible operative mortality and morbidity and longer hospital stays than primary mitral surgery. In addition, during the second operation, the valve is often replaced, for a variety of reasons: reluctance of the patient and/or the surgeon to take the risk of a second failure, anatomical complexity and tissue properties. Percutaneous interventions such as valve-in-valve or valve-in-ring interventions are appropriate in some of these cases; however, the procedure is not always feasible because of the risk of LVOT obstruction, paravalvular leak and potential embolisation. TEER is a promising alternative strategy to redo-surgery, aiming to stabilise the previous repair and avoid valve replacement (Figure 3). Imaging challenges brought on by prior valve surgery and the gradient following mitral intervention should be taken into account, although the presence of a small ring is not necessarily a contraindication to the procedure. The regurgitant jet width and the amount of residual tissue also play a major role in predicting postprocedural mitral gradients. Braun et al retrospectively collected data on 57 patients treated in 11 centres from 2010 to 2016 who underwent TEER after a previous mitral valve repair. Acute procedural success (postprocedural MR ≤2) was achieved in 84% of patients, but it was limited by imaging issues among patients with annuloplasty rings and the development of mitral stenosis31. A recent international, multicentre, observational, retrospective study, which included 104 patients after failed surgery, showed that TEER was feasible and safe in selected patients, with technical and device success rates of 90%. However, the authors suggested that additional/modified imaging techniques may facilitate leaflet grasping and shorten device time by dealing with technical challenges caused by shadowing from the annuloplasty ring32. Furthermore, it should be highlighted that TEER has been attempted in selected populations in experienced centres, and therefore, careful patient selection in high-volume centres is warranted.
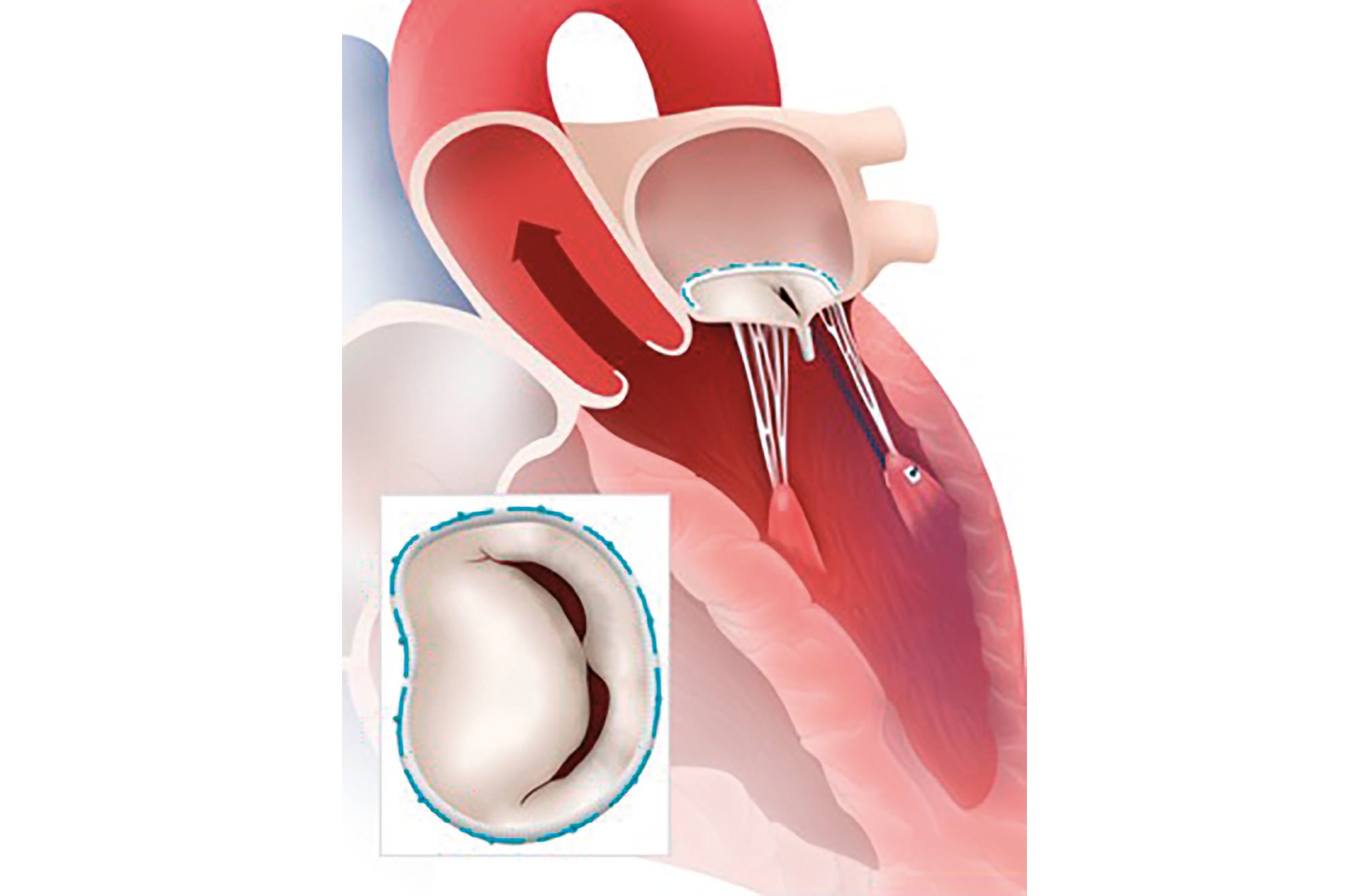
Figure 3. TEER in failed surgical mitral intervention. A ruptured chord in a previous surgical mitral ring implantation treated with TEER in a ring. TEER: transcatheter edge-to-edge repair.
TEER in complex mitral valve anatomy
The landmark randomised Endovascular Valve Edge-to-Edge Repair Study (EVEREST) II set anatomical criteria for successful TEER: the optimal anatomy is when the primary regurgitant jet originates from malcoaptation of the A2 and P2 scallops of the mitral valve, with sufficient leaflet size and no large flail gaps33.
Over the years, the TEER technique has gained popularity with increased procedural success in more complex anatomies. The use of newer devices, such as the fourth-generation MitraClip or the PASCAL Ace (Edwards Lifesciences), and the emergence of three-dimensional (3D) transoesophageal imaging has allowed the operators to choose the device size, or combination of devices, based on each patient’s mitral valve anatomy. The ability to perform independent leaflet grasping has allowed for treatment of more challenging mitral valve anatomies including wide coaptation gaps, large redundant tissue, and larger flail gaps that were far beyond the initial EVEREST II criteria34. Recent data evaluated 386 patients who were treated with TEER, of whom 70 patients (18%) had been considered unsuitable for TEER because of prior valve surgery, a small mitral valve area, type IIIa morphology, a larger coaptation depth, and a shorter posterior leaflet. In 70% of the unsuitable patients, TEER decreased MR without any adverse events, and 1-year survival with mild or no symptoms was 52%35. The PASCAL IID registry specifically evaluated the effectiveness of the PASCAL device in complex degenerative mitral disease, including bileaflet prolapse and a large flail gap. The implant success rate was 92.9%, and, at 6 months, MR ≤2+ was achieved in 92.4% of patients and MR ≤1 was achieved in 56.1%36. Two prospective randomised clinical trials are currently enrolling patients with symptomatic primary MR. The REPAIR MR (Percutaneous MitraClip Device or Surgical Mitral Valve REpair in Patients With PrimaRy Mitral Regurgitation Who Are Candidates for Surgery) trial aims to assess the outcomes of TEER versus surgical repair for severe primary MR in older and/or moderate surgical risk patients. This prospective, randomised, multicentre trial, with 500 eligible subjects, will compare the 2 treatments to establish the non-inferiority of TEER for the treatment of severe primary MR37. Conversely, the PRIMARY trial (ClinicalTrials.gov: NCT05051033) is a prospective, randomised study enrolling 450 patients aged 65 or older with primary degenerative MR, comparing TEER with surgical repair within this demographic. Both trials are expected to significantly influence MR treatment recommendations and clinical decisions.
However, there are still limitations of performing TEER in specific anatomies. A consensus document on non-suitability for transcatheter mitral valve repair by edge-to-edge therapy recommended avoiding TEER in patients with chronic rheumatic disease, severe mitral annular calcification with mitral stenosis, and a prohibitively small mitral valve area (<3.5 cm2), as well as when the MR is primarily due to cleft anatomy38.
Various transcatheter mitral valve replacement (TMVR) devices are being developed and tested in clinical studies concurrently. In situations where TEER is not an option due to complex mitral anatomies, these devices with different anchoring mechanisms, delivered by either transapical or transseptal approaches, may be used in future to replace the mitral valve. The most notable benefit of TMVR over transcatheter repair is a greater and longer-lasting reduction in MR39. However, TMVR screening failure is currently very high. In fact, Niikura et al suggested that only 10% of patients who were screened were eventually treated with TMVR. In that trial, the most common reasons for exclusion were excessive frailty, severe tricuspid disease and prior aortic valve therapy. Mitral anatomical exclusions included severe annular calcification and risk of LVOT obstruction40. For this reason, patients with a suboptimal anatomy for TEER are sometimes treated in high-volume centres by expert operators. In future, a complementary approach between replacement and repair is desirable. The decision between TMVR and TEER hinges on a variety of critical factors. These factors encompass the anatomical considerations that dictate the appropriateness of each intervention, such as the potential for substantial residual MR and/or mitral stenosis. Equally important are the clinical parameters, the patient’s overall health, their associated risks, and their individual preferences, all of which necessitate thorough evaluation.
For example, elderly patients with comorbidities and a challenging valve anatomy might be more suited to the TEER approach, even when anticipating a less-than-perfect reduction in MR, as it may still lead to clinically meaningful improvement. On the other hand, younger patients with fewer comorbidities but still at high surgical risk should be evaluated and considered for TMVR.
TEER in congenital heart disease
Congenital heart disease typically affects younger adults who are at higher risk for surgical complications and have had prior open-heart surgery. Still, there are no percutaneous devices currently approved for the treatment of atrioventricular valve regurgitation (AVVR) in adults with CHD. For symptomatic mitral and tricuspid regurgitation in patients with CHD deemed ineligible for surgery, TEER might be a viable technique. Patients with CHD may have a complex anatomy and may have undergone previous surgeries that can make the TEER procedure more challenging. Patients with a systemic right ventricle (SRV), particularly those with congenitally corrected transposition of the great arteries (ccTGA) and those with atrial corrected transposition of the great arteries who have not undergone surgery, can develop AVVR and SRV dysfunction which often impact morbidity and mortality.
In some cases, AVVR may be treated with percutaneous devices. These procedures are usually performed in the SRV − the right ventricle in patients with CHD. In patients with ccTGA, the atrioventricular valve is trileaflet, and therefore, TEER might be more complex than conventional mitral interventions. In patients who underwent atrial surgical interventions such as the Senning or Mustard procedures, there is a need to cross the baffle in order to reach the valve. Given the significant anatomical differences, it is challenging to extrapolate these procedures from common degenerative atrioventricular valve disease to CHD. For example, procedural planning often requires advanced imaging such as computed tomography and magnetic resonance imaging. Three-dimensional printed models might also be beneficial for screening, planning and performing TEER. They enable precise, patient-specific planning and provide an opportunity for hands-on simulation before TEER. Additionally, specific procedural techniques often need to be modified. Franzen et al emphasised the need for a higher position than the mitral annulus when performing the transseptal puncture, a more medial position during device steering, and an altered orientation angle for clip positioning41.
TEER appears to be a safe therapy in patients with CHD; however, most publications are anecdotal with limited long-term data. Further investigation is needed to determine the safety and effectiveness of these procedures42.
Potential future indications for TEER
In current practice, the decision between surgical or percutaneous mitral valve interventions is based on the risks versus benefits of open surgical valve intervention, which, for years, has been the standard of care. As TEER is minimally invasive, safe, and has rapid recovery times, it is possible to consider TEER in earlier stages of mitral valve disease.
This approach may be appropriate for various patient subgroups in diverse clinical settings. For example, in primary MR, the indications for intervention are determined by the presence of symptoms or the progression of untreated MR-related complications, like LV dysfunction or dilatation, pulmonary hypertension, or atrial fibrillation. It is plausible that early TEER in asymptomatic patients might prevent MR-related complications. Similarly, the potential role of TEER for patients with mild symptoms due to ischaemic MR soon after MI is currently undetermined. These patients are typically managed medically, as they are often poor candidates for standard surgical procedures. Nevertheless, performing TEER at this stage could potentially mitigate the adverse effects of severe MR following MI and prevent unfavourable LV remodelling. In ischaemic MR animal models, only early MR repair (up to 30 days after experimental MI) prevented adverse LV remodelling, suggesting a point of no return after which the beneficial effect of MR repair is limited43. However, no data are available to support early TEER in stable post-MI patients and further research is needed to evaluate its effectiveness in this population.
Another group that might benefit from mitral intervention are patients with atrial myopathy and associated atrial fibrillation44. Tamargo et al evaluated patients with HF and preserved LV with and without MR. They found that patients with MR displayed greater LA volumes, reduced LA strain and compliance, and greater mitral annular dilatation. They concluded that SMR reflects LA myopathy and is linked to worsened haemodynamics, disease severity, and reduced functional capacity45. The impact of early mitral intervention on atrial myopathy and on the atrial fibrillation burden has not yet been evaluated. Suggestions of possible future clinical trials are presented in Table 1.
Table 1. Possible clinical trials.
| Hypothesis | Study population | Intervention | Outcomes of interest |
|---|---|---|---|
| Early intervention in patients with primary MR might improve clinical outcomes and prevent MR-related complications. | Asymptomatic patients with primary severe MR who are at high risk for surgical mitral valve repair with anatomical suitability for TEER | TEER versus medical therapy | Clinical outcomes, mortality, hospitalisations, AFib burden, and pulmonary artery pressures |
| Early intervention in post-MI MR might improve clinical outcomes and prevent adverse LV remodelling. | Symptomatic patients with severe MR within 90 days after acute MI | TEER versus medical therapy | Clinical outcomes, mortality, hospitalisations, and LV remodelling parameters |
| Mitral intervention among patients with atrial myopathy might prevent adverse LA myopathy and remodelling. | Patients with atrial myopathy and paroxysmal AFib and secondary severe atrial MR | TEER versus medical therapy/AFib ablation | Clinical outcomes, mortality, hospitalisations, LA remodelling parameters, and AFib burden |
| AFib: atrial fibrillation; HF: heart failure; LA: left atrial; LV: left ventricular; MI: myocardial infarction; MR: mitral regurgitation; TEER: transcatheter mitral edge-to-edge repair | |||
Conclusions
The edge-to-edge repair method remains the most versatile technique, able to address a wide range of causes and mechanisms of atrioventricular valvular regurgitation. Due to its safety profile and versatility, physicians continue to explore clinical applications beyond the limits of the current evidence. As a consequence, TEER has become an important complementary solution to other treatments in patients previously deemed inoperable or at high risk. While the effectiveness and safety of the TEER technique in these scenarios have been reported in several case reports and case series, its role has not been determined conclusively, and further prospective data are needed. In the meantime, the availability of a simple and safe method to manage valve regurgitation offers a solution for many patients who might otherwise remain untreated.
Conflict of interest statement
M. Shuvy is a clinical proctor for Abbott. F. Maisano has received grants and/or research institutional support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific, NVT, Terumo, and Venus Medtech; has received consulting fees, and personal and institutional honoraria from Abbott, Medtronic, Edwards Lifesciences, Xeltis, Cardiovalve, Occlufit, Simulands, Mtex, Venus Medtech, and Squadra; has received royalty income/IP rights from Edwards Lifesciences; and is a shareholder (including share options) of CardioGard, Cardiovalve, Magenta, SwissVortex, Transseptal Solutions, 4Tech, and Perifect.

