Introduction
It is well known that cardiologists, in the field of invasive diagnostic angiography and percutaneous interventions, are among the most frequent users of fluoroscopy in the medical world. Recent attention to the high radiation doses utilised in other cardiac imaging modalities such as nuclear and computed tomographic angiography has increased awareness of this important issue. Interventional cardiology currently accounts for at least 50% of total effective dose by radiation used in medical imaging in Europe and in the United States1,2. While there has been recent attention to this issue by the scientific and lay press, along with increasing regulatory scrutiny, many cardiologists have been slow to adopt better practices to reduce radiation exposure to their patients and themselves. The short and long term risks of excessive radiation exposure are well known. Excessive patient radiation exposure from fluoroscopy in the United States must now be reported as a sentinel event as mandated by the Joint Commission or Accreditation of Healthcare Organizations (JCAHO)3.
The aim of this brief contribution is to review and describe the different tools and techniques available to decrease radiation exposure and risk in the cardiac catheterisation laboratory and
to suggest additional strategies and improvements in technology. We have attempted to make this document easy to understand and to be of practical value for invasive cardiologists, regardless of the depth of their knowledge of radiation physics.
Definitions, measurement units and radiation risks
For the purpose of this review, the emphasis has been placed on patient exposure and risk. However, it is a reasonable assumption that by decreasing radiation exposure to the patient, exposure to the operator and attending personnel will also be reduced. Radiation exposure to radiation workers (principally by scattered radiation from cardiac fluoroscopy) is described in terms of effective dose and equivalent dose in the International Commission on Radiological Protection report 60 (ICRP)4. The effective dose (ED) is a weighted sum of equivalent doses delivered to various organs and is used to assess the stochastic risk (no threshold dose and includes cancer and genetic mutations)5. This is typically estimated using the readings from radiation badges and is reported in units of mSv6. Maximal annual permissible levels among radiation workers are stated in most countries. Dose to professionals as cardiologist in the range of 20 mSv correspond to an elevated risk of death by cancer of 1/10007.
Radiation risks to patients are of two types: stochastic (as above and usually long term) and deterministic (threshold dose leading primarily to skin burns and ulceration and occurs early). Although stochastic risks are difficult to predict, growing concern of their risks lies with increasing numbers of procedures performed on younger patients, multiple and complex procedures, all with risks that go unnoticed because procedures are often associated with doses that are below the deterministic threshold. In high dose ranges, the severity of the effect is dose and organ dependent. These deterministic effects in the case of fluoroscopy are related to the skin dose. Thus the dose delivered locally to the skin should be minimised to prevent burns (ranging from a transient erythema to skin necrosis) and more serious problems.
To evaluate and hopefully limit this deterministic risk, cardiologists require an estimate of the highest dose delivered to the skin (entrance skin dose). Unfortunately it is impractical to measure this directly in routine clinical practice. Instead, an estimate of this dose can be provided by the “Reference Point Dose” (RPD) available from current imaging systems which is reported in Gray (Gy) or mGy units. This is also referred to in a practical sense as the “cumulative dose” and is measured at a fixed reference point from the isocentre towards the X Ray tube8. It is only an estimate of the local skin dose since actual skin local dose will depend on distribution of the dose during the procedure as determined by different gantry angulations. All of these data can be collected and analysed off-line following the procedure. It is this measurement that the FDA has mandated be used to trigger a sentinel event report (currently 15 Gy).
The other commonly available measurement is the dose-area product (DAP) or kerma-area product (Gy.cm2)9. This is an important measurement to look at in terms of control of the stochastic risk. The most important factors influencing DAP are field size, fluoroscopy level, use of cine-angiography, complexity of the procedure, and operator skill - all of which are under the control of the operator10.
Therefore, cardiologists currently can limit risk of short term complications of their patients by following the RPD during the procedure. In addition, monitoring the DAP allows benchmarking of their practice and providing target dose limits in an effort to reduce the long term effects associated with use of fluoroscopy.
Steps to limiting radiation exposure
Most steps to limiting the amount of radiation exposure to patients and operators are well documented, but adherence to these by cardiologists is highly variable.
A) Equipment: modern and well maintained and calibrated fluoroscopic systems are essential. Modern systems provide pulsed progressive fluoroscopy and choices of dose and frame-rate11. Options also include ability to store fluoroscopic images, thus avoiding the need to repetitively perform cine-angiography during PCI. Appropriate filtration should be used along with easy-to-use collimators. Adequate shielding above and below the table should always be provided and used12.
B) Operator practice: The operator should be knowledgeable of the equipment’s features designed to limit radiation exposure such as adjusting frame-rate, using collimators, avoiding unnecessary magnification, etc. Minimising cine-angiographic (digital acquisition) runs, minimising total fluoroscopic time, ensuring the image detector-to-patient distance is as low as possible and fluoroscopic tube to patient distance as high as possible, avoiding unnecessary steep angulated views (particularly in the left anterior oblique), limiting use of high dose fluoroscopy (vascular access: femoral versus radial13,14 approach), awareness of situation and attending personnel, and remembering the “inverse-square rule”. Use of lead aprons and eye protection should be routine15.
C) Patient factors: Increasing thickness and body weight are key contributors to total radiation dose delivered. Morbidly obese patients present challenges to the equipment and the operator; poor image quality (with temptation to consider using even higher doses), prolonged procedures and more scatter to the operator can be anticipated. The operator needs to account for these factors when planning and performing these procedures in such patients6.
Further strategies to decrease radiation exposure
While the above points are deserving of continuing emphasis, newer strategies are also warranted. There is an urgent need for real-time monitoring and alert system to the operator of the RPD: this is a relatively new concept. Some labs now routinely make the operator aware via verbal alerts that threshold RPD limits have been approached or exceeded (e.g. 3 Gy followed by 6 Gy and higher). This has obvious benefits in a system in which exceeding certain limits will initiate reporting of the event to the local radiation safety office or to state regulatory agencies. There is a need for industry help to improve the visibility of display and alerts of RPD and DAP measurements in real-time. Perhaps systems could be designed to automatically vary angulation, collimation and dose once excessive exposure in an unchanged gantry position has been detected.
The complexity of the procedure is also a critical factor in the cumulative dose measurement. Highly complex procedures such as treatment of bifurcation lesions and chronic total occlusions can be associated with very high exposure to radiation. Appropriate planning of the procedure with an emphasis on strategies to decrease the amount of x-ray exposure should be strongly encouraged. Additional complicating factors such as patient obesity, and difficult vascular access etc should also be taken into account. Complexity indexes have been described16 but will need further exploration with validation in large numbers of patients and in individual labs.
Patient follow-up
Clinical follow-up of any patient identified as having been exposed to excessive radiation exposure should be arranged. An explanation of the dose measurement and why it was so high should be given to such patients along with instructions on what to look for with respect to erythema and/or burns. Routine clinical re-evaluation and examination should be arranged for these patients over the next few weeks. Referral to a dermatologist should also be made whenever there are signs of significant burns.
Quality assurance and education
It would be helpful if regular feedback was provided to operators with their RPD and DAP measurements of their patients, with comparison to their colleagues. Cumulative radiation dose for each patient should be recorded and stored in each lab and consideration also made to adding this to the medical record. Each lab could then set goals and strategies for reducing the total amount of radiation exposure.
New cardiology trainees, particularly those in interventional training programs, require more focussed practical education of radiation exposure and safety issues17. Importantly, we as practising specialists need to set a better example as we teach these operators of the future. Continuing education in this field should be mandatory; more sessions – educational and research – should be devoted to this entire field at major cardiology meetings.
Practical hints
One of the main issues in the daily practice of the interventionist is to establish a practical guideline for the management of patient who underwent procedure in interventional cardiology. to our knowledge, there is currently no standard «radiation risk score» in interventional cardiology correlated to a clinical follow up of the patients. The Tables 1 and 2 introduce the concept of a suggested radiation clinical management exposure score (RCME) correlated with a practical clinical follow-up guideline.
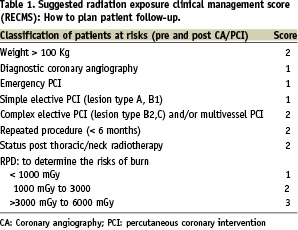

This proposed score can used in daily practice of cardiac percutaneous procedure. In addition we summarised practical recommendations in Table 3 to optimise radiation protection of the patients, staff and operator.

Conclusion
The key message of this contribution is that no safe dose exists and we must assume that there is a need for improvement in the practice of radiation protection. The linear, no-threshold dose-effect relationship adopted by the international community is a convenient and broadly accepted tool. Finally, the application and the respect of simple rules as described in this discussion will increase the protection of the patient and all the staff present in the catheterisation laboratory.

