Introduction
Structural valve deterioration (SVD) is the most frequent long-term complication of implanted bioprostheses (BP). Redo prosthetic valve replacement has remained, until now, the sole effective treatment for severely deteriorated bioprosthetic valves, but this procedure carries an increased operative risk in comparison to the first operation1. With the broadening of the indication of bioprosthetic valve replacement, more and more cases are expected to occur in the future, particularly in elderly patients. Endovascular valve implantation (VI) might be a valuable alternative to conventional surgery in this high risk population.
Endovascular VI has already been performed in patients suffering from aortic stenosis or pulmonary insufficiency using either balloon expandable or self-expandable valved stents2-4. A major problem encountered with percutaneous VI is the impossibility to readjust the position of a valved stent (VS) once fully deployed. Bad positioning of the VS could lead to a fatal peri-procedural issue3. None of the available VS has the potential to be repositioned after complete deployment5. Thus, being able to reposition a fully deployed VS could prove of crucial importance and would certainly favour the development of percutaneous VI by improving the safety of the procedure.
Methods and results
We have evaluated an original ancillary system allowing a self-expandable VS to be repositioned as often as necessary during the procedure. The prototype consisted in a semi-rigid delivery catheter made of polyvinyl chloride. Two sutures ran along the internal lumen of the delivery catheter. At their distal part, these two sutures encircled the VS, thus allowing its attachment to the tip of the catheter (Figure 1).

Figure 1. Valved stent attached to the tip of the delivery catheter (note the presence of 2 encircling sutures).
At their proximal part, the sutures were attached to a handle. Traction or relaxation of the handle was associated to compression or relaxation of the VS (video 1). Once deployed in adequate position, the VS was definitely released from the delivery catheter by pulling on a wire that controlled the attachment of the two sutures. The delivery catheter could then be withdrawn leaving the VS in place.
We developed an animal model of tricuspid bioprosthetic failure in sheep. This consisted in surgical implantation of a pericardial bioprosthesis, the anterior leaflet of which was torn. Severe tricuspid regurgitation was well documented in each case (n=5). Endovascular VI of the failed bioprosthesis was easily achieved in all cases (100% success rate). The procedure took no more than two minutes and was haemodynamically well tolerated. The repositioning capacity of the delivery device was confirmed in all experiments with no failure (video 2).
Bioprosthetic failure was always corrected, with no residual intra- or periprosthetic leak on echocardiography. Anatomic study revealed the excellent location of the VS inside the failed BP (Figure 2).
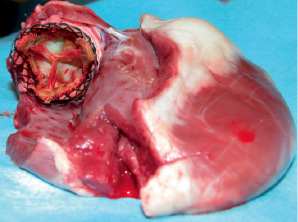
Figure 2. Apical view of the heart (the right atrium and the anterior part of the right ventricle have been resected) showing excellent positioning of the stented valve inside the failed bioprosthesis.
No macroscopic evidence of injury to the VS was noticed.
In order to achieve adequate positioning of the VS, right ventricular pacing or use of extracorporeal circulation during VS delivery has been recommended by others. With the present device, these potentially deleterious strategies are likely to be unnecessary, as it was the case in our experimental study. This original delivery device permitted the reversibility of the VS positioning as many times as needed prior to the final release. This relied on a simple manoeuvre (compression-relaxation of the VS) that could be performed by one operator only.
The VS we used in this experiment was made of a self-expandable braided nitinol stent. A porcine aortic stentless valve (Toronto SPV, model SPA-101-25, St Jude Medical, Minneapolis, MN, USA) was fixed to the stent using 4/0 mattress sutures. The design of the delivery system allows other VS (with pericardial or polymeric leaflets, for example) to be used provided that their stent is self-expandable. The number of the encircling sutures will mainly depend on the stent length, but should be as low as possible (one or two) for easiest handling.
Conclusion
Although our experimental investigation was limited to the treatment of bioprosthetic failure, this new technology is easily applicable to the treatment of other valvular diseases, such as pulmonary insufficiency or aortic stenosis. One may also anticipate that the safety improvement conferred by this new technology will certainly favour the development of percutaneous VI in clinical practice.
Online data supplement
Video 1. Compression or relaxation of the valved stent
Video 2. Location of the VS inside the failed bioprostheses

