Introduction
Stent thrombosis (ST) carries with it substantial morbidity and mortality1,2. The identification of clinical, lesion, and procedural risk-factors (RF) that are associated with ST may improve individual risk stratification3. The additional role of platelet reactivity as a risk factor, although appealing, is still in an investigational phase. In the present review we discuss the currently available evidence on platelet reactivity as a risk-factor for stent thrombosis.
The vascular response to stenting
Under physiological conditions the intact endothelium plays a key role in the regulation of thrombosis and haemostasis. Paradoxically, endothelial cells secrete both multiple prothrombotic substances (such as tissue factor and von Willebrand factor) as well as antithrombotic molecules (such as nitric oxide and prostacyclin). When the endothelial barrier becomes injured (e.g., as a results of balloon angioplasty or coronary stent implantation), the modulating capacities of the endothelium disappear and the subendothelial extracellular matrix and lipid core components become exposed to the flowing blood4. The role of platelets in response to the iatrogenic induced endothelial injury, or to the implanted (prothrombotic) coronary stent is well recognised and can be divided in three consecutive phases (Figure 1): adhesion (A), activation (B) and aggregation (C)5.
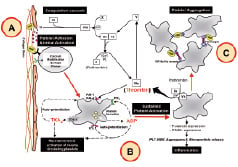
Figure 1. Coagulation cascade: Exposure of tissue factor after vascular injury (A) leads to complex formation with factor VIIa. The TF-VIIa complex cleaves factor X to factor Xa. Factor Xa in the prothrombinase complex activates prothrombin (factor II) to thrombin (factor IIa). The activation of thrombin occurs on the surface of activated platelets. Platelet phase: Upon vascular injury (A), platelets adhere to exposed proteins (such as collagen, von Willebrand factor) and are activated via several independent mediators (B). Once activated, platelet release secondary agonists (such as ADP and thromboxane A2), that in turn recruit additional platelets to aggregate with one and another via GP IIb/IIIa mediated fibrinogen cross-linking (C). ADP: adenosine diphosphate, Ca2+: calcium, COX: cyclo-oxygenase activity, PL: phospholipids, PLT: platelet, TF: tissue factor, TXA2: thromboxane A2, vWF: von Willebrand factor, WBC: white blood cells
Adequate antithrombotic therapy during and after PCI is therefore of utmost importance, and the currently recommended strategy with dual antiplatelet therapy has proven to be highly effective in the prevention of stent thrombosis until the stent struts are covered with endothelium. Despite antiplatelet therapy, however, it has become clear that a substantial number of patients still suffer from recurrent ischaemic coronary events6.
Evidence is mounting in recent years that besides the already recognised clinical, lesion and procedural factors, a persistent heightened (residual) platelet reactivity – despite the use of aspirin and clopidogrel – may also be an important determinant of stent thrombosis. This crucial evidence is derived from multiple sources:
1) Intensified antiplatelet therapy decreases the incidence of stent thrombosis7.
2) Premature discontinuation of antiplatelet therapy increases the risk for stent thrombosis2,8.
3) Autopsy reports describe platelet deposition in the stent-area9.
4) Platelet function studies have suggested a causal relationship between the absolute magnitude of platelet reactivity and the occurrence of stent thrombosis10,11.
In this review, we discuss the contribution of platelet reactivity to the phenomenon of stent thrombosis and we briefly review the available techniques to measure platelet reactivity.
Wide variability in the response to antiplatelet therapy
In the last years, multiple studies have demonstrated that the response to a fixed dose of antiplatelet therapy (clopidogrel and aspirin) is highly variable and the responsiveness to clopidogrel as measured with ADP-induced aggregation follows a bell-shaped Gaussian-distribution6. Consequently, a substantial proportion of patients receiving combination therapy with aspirin and clopidogrel fail to obtain optimal benefit from it. This phenomenon has been termed “resistance to antiplatelet therapy” in the medical literature12,13. However, the somewhat confusing term “resistance” implies that these drugs do not reach its pharmacological target at all, which is not the case in most instances. Hence, alternative definitions have been recently introduced (Figure 2)14.
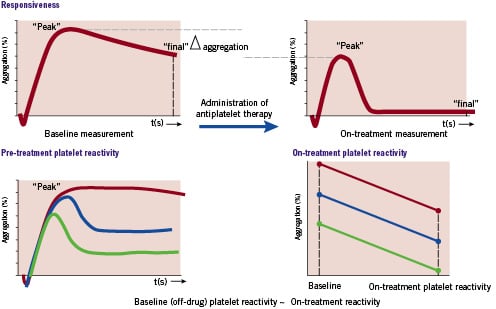
Figure 2. Platelet aggregation curves to illustrate the essential difference between the three definitions. Upper part: responsiveness to antiplatelet therapy. Lower part: Pre- and on-treatment platelet reactivity.
Responsiveness to antiplatelet therapy
The individual responsiveness to antiplatelet therapy can be determined when platelet function is evaluated at two time points: before the administration of the antiplatelet drug and after the antiplatelet drug has reached its maximum effect. The absolute difference in the degree of platelet reactivity before and after the administration of antiplatelet therapy is defined as responsiveness.
Pre-treatment platelet reactivity
Evidence strongly suggests that individuals vary in their platelet response to pro-activating stimuli (e.g., vascular injury, atherosclerosis, adenosine diphosphate, thromboxane A2) and thus in their thrombotic potential.15 Consequently, a subgroup of individuals exhibit hyper-reactivity and this increases the likelihood for the occurrence of an atherothrombotic event. The absolute magnitude of the individual’s intrinsic baseline (off-drug) platelet reactivity is called: pre-treatment platelet reactivity.
On-treatment platelet reactivity
The absolute magnitude of (residual) platelet reactivity that is measured in patients receiving antiplatelet therapy is defined as on-treatment platelet reactivity (also referred to as residual platelet reactivity).14
Low responsiveness, as well as high on-treatment platelet reactivity, has been linked to adverse clinical outcome. However, it remains unclear which parameter is the most predictive. In an attempt to answer this difficult question, Samara and co-workers have recently posted the following statement: “Measuring clopidogrel responsiveness may overestimate the risk of stent thrombosis in non-responders with low pre-treatment reactivity and underestimate risk in those responders who remain with high on-treatment platelet reactivity. Therefore, high on-treatment platelet reactivity is a better measure of thrombotic risk than responsiveness to clopidogrel.”16
Platelet reactivity as a determinant of stent thrombosis
Several studies have suggested that some – but not all patients who eventually develop a stent thrombosis – exhibit high on-treatment platelet reactivity. The initial evidence for this hypothesis was derived from a number of retrospective case-control studies (Table 1)17-21.
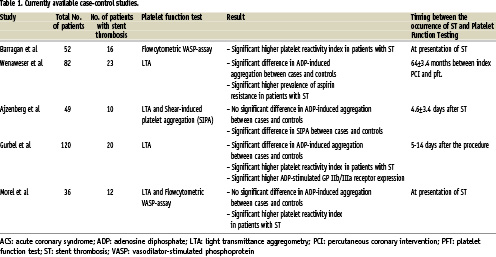
It is important to note that these studies are relatively small and yield conflicting results: some indicate significant differences in the magnitude of ADP-induced aggregation between patients with ST and controls, whereas others report no differences at all. A likely explanation for this apparent contradiction appears to be due to differences in the timing of platelet function evaluation (in the acute phase of the ST versus later on, when platelet activation is normalised), differences in control group selection and the use of different platelet function assays.
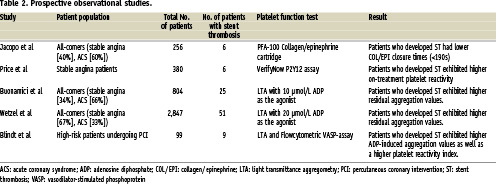
Results from prospective observational studies also support the hypothesis that patients with high on-treatment platelet reactivity despite aspirin and clopidogrel therapy are more likely to suffer from a stent thrombosis (Table 2)11,22-25. The problem with these studies is the relatively low number of events and the diversity in definitions (thresholds) to identify those patients who are at an increased risk for ST.
Methods to determine platelet reactivity
An abundant variety of techniques is currently available to monitor platelet function in patients receiving antiplatelet therapy (Table 3)26-30. However, given the very complex nature of platelet biology, there is no platelet function test that covers all aspects. Moreover, the answer to “which is the right test to measure platelet function?” varies according to the purpose of testing. Several commercial “point-of-care” platelet function assays have been designed with the specific purpose of monitoring different types of antiplatelet therapy. As a consequence, these assays can be used to answer the question: “does the aspirin or clopidogrel therapy actually work in my patient”. Nonetheless, the residual magnitude of platelet reactivity can still be heightened despite an adequate effectiveness of antiplatelet drugs. Likewise, other platelet function assays have been developed to screen the general platelet reactivity status of a patient without knowing the individual effects of antiplatelet drugs.
Currently available platelet function assays
The following assays are currently available for the monitoring of the individuals response to antiplatelet therapy in the catheterisation laboratory. The advantages and shortcomings of these methods as well as their potential role for possible application in the catheterisation laboratory are highlighted.
Light transmittance aggregometry
The most commonly used method to evaluate platelet function has been the optical detection of platelet aggregation in platelet-rich plasma (PRP) termed light transmittance aggregometry (LTA).31 A sample of citrated blood is collected and centrifuged to obtain PRP. After removing the platelets in the centrifuged tube, platelet-poor plasma (PPP) is prepared from the remaining sample to serve as the 100% aggregation reference. PRP is then pipetted in a cuvette and is warmed to 37 °C and light transmission through the PRP is detected by a photometer. Various agonists can be added to the cuvette to stimulate specific platelet activating-pathways: e.g., ADP, the agonist to evaluate the effects of clopidogrel; arachidonic acid, the agonist to evaluate the effects of aspirin, collagen and/or thrombin-receptor activating peptide (to quantify magnitude of the general platelet reactivity of an individual). Upon addition of an agonist, the platelets change from their disc shape to a more rounded form with pseudopods, resulting in a transient, small decrease in light transmission that is followed by an increase in light transmission as the platelets aggregate. Parameters measured include the slope of aggregation, the measurement of aggregate stabilisation and the maximal amplitude or percentage of aggregation after a fixed period of time.
ADVANTAGES OF LTA
LTA is available in almost every laboratory and has been in use for more than 35 years. Additionally, multiple studies (despite arbitrarily chosen cut-offs to segregate responders from non-responders) have shown that LTA is capable of predicting the occurrence of atherothrombotic events.
SHORTCOMINGS OF LTA
LTA has several serious drawbacks that make it impossible to incorporate LTA into the routine practice. These include: poor reproducibility which makes comparison between laboratories difficult, a long sample processing time and the expertise of specialised technicians. Furthermore, many factors such as platelet activation by centrifugation, sample ageing and the presence of other substances (e.g. lipids) may affect the magnitude of platelet aggregation. Another limitation of LTA is that it is based on fibrinogen-GP IIb/IIIa mediated aggregation. However, under physiological conditions (e.g., under the high shear rates of arterial flow) the interaction between the GP IIb/IIIa receptor, vWF and subendothelial components (e.g. collagen) are at least of equal importance in the process of thrombus formation.
The VerifyNow™ system
The VerifyNow system (formerly known as the Rapid-Platelet Function Analyzer or Ultegra; Accumetrics, San Diego, USA) is based upon the principle that agonist-induced activated platelets bind to fibrinogen-coated polystyrene beads that agglutinate in whole-blood26. After whole blood is added in the mixing chamber, the platelets become activated by a specific agonist if the antiplatelet drug does not adequately exert its antiplatelet effect. As a result, the platelets bind via GP IIb/IIIa receptors to the fibrinogen-coated beads and cause agglutination. Infra-red light transmittance through the chamber increases as the platelet-beads complexes fall out of the solution. Currently, three different cartridges are available: the VerifyNow™ aspirin assay (using arachidonic acid [AA] as the agonist), the VerifyNow™ P2Y12 assay (using ADP as the agonist, antagonised by prostaglandin E1) and the VerifyNow™ IIb/IIIa assay (using thrombin receptor-activating peptide [TRAP] as the agonist).
ADVANTAGES OF THE VERIFYNOW™ SYSTEM
It is a bedside device (really “point-of-care”) that provides simple, rapid and accurate results. Although relatively small, several studies have indicated that the results obtained from any of the three assays correlate with the (re)occurrence of cardiovascular events.
SHORTCOMINGS OF THE VERIFYNOW™ SYSTEM
In contrast to other commercial platelet function assays, the VerifyNow™ assay differs from in vivo conditions because it does not assess platelet function under high shear conditions. In addition, the VerifyNow™ assays are relatively expensive when compared to the other available platelet function assays.
The Platelet Function Analyzer™ (PFA-100)
The PFA-100® Analyzer (Dade Behring Marburg GmbH – A Siemens Company, Marburg, Germany) measures platelet function, in particular adhesion and aggregation, in whole blood under high shear conditions (thereby mimicking the pressure in a capillair in the human body)27. Specifically, the time needed to form a platelet plug occluding the aperture cut into a collagen/ epinephrine (COL/EPI) or collagen/ADP (COL/ADP) coated membrane is determined and reported as closure time (CT) in seconds. If the formed clot is too weak, the CT can not be measured and the results are presented as >300 seconds.
ADVANTAGES OF THE PFA-100™ SYSTEM
The instrument uses whole blood, the technique is easy to learn and is semi automated. The assay evaluates both shear-stress and fibrinogen-induced GP IIb/IIIa-mediated aggregation, which corresponds better with the in vivo process of thrombus formation. Also, the results obtained of the COL/ADP provide a general view of the individual’s magnitude of platelet reactivity. As a result, there is some evidence that has linked shorter COL/ADP closure times with a higher (re)-occurrence of atherothrombotic events.
SHORTCOMINGS OF THE PFA-100™ SYSTEM
It has been estimated that the concentrations of collagen and ADP are far beyond the physiological concentrations of these substances in vivo. Hence, it has been suggested that clopidogrel is not able to inhibit the platelet plug formation under these high concentrations of collagen and ADP in the cartridges. A new cartridge with the specific purpose to monitor the inhibitory effects of clopidogrel is currently under development.
The Plateletworks® system
The PlateletWorks® system (Helena laboratories, Beaumont, USA) is based on a single platelet counting principle, expressed as the platelet count ratio before and after exposure to ADP, to calculate the percentage of platelet inhibition30. A total reference platelet count is performed in whole blood in a Plateletworks® tube containing K3-EDTA as the anticoagulant on a cell counter. This process is repeated with a second sample of fresh whole blood in a Plateletworks® tube containing both D-Phe-Pro-Arg-chloromethylketone (PPACK) 50 µmol/L and ADP, AA or collagen. In the presence of a the agonist, platelets associate and aggregate. As the aggregated platelets exceed the threshold limitations for platelet size, they are no longer counted as individual platelets. The ratio of the platelet count between the agonist tube and reference tubes is expressed as percentage of inhibition of platelet aggregation (IPA).
ADVANTAGES OF THE PLATELETWORKS®
The method is simple, inexpensive, quick to perform (5 minutes) and thus easy to incorporate into daily practice.
SHORTCOMINGS OF THE PLATELETWORKS®
Measurements should be performed within ten minutes after the addition of 1 ml of whole blood. Thereafter, ADP-induced platelet aggregates will dissociate and the extend of platelet inhibition will be overestimated (van Werkum, unpublished data). Also, it has been shown that the Plateletworks® assay measures lower levels of IPA as compared to LTA. This has led to the hypothesis that LT aggregometry provides information regarding the platelet macro-aggregate formation only, while the platelet count ratio measures the platelet macro- and micro- aggregate formation. The relevance of micro-aggregation as predictor for clinical outcome, however, has not been established.
Conclusion
The observed wide inter-individual variability in the response to aspirin and clopidogrel therapy has increased the interest in the use of platelet function assays. Moreover, the body of evidence showing that heightened platelet reactivity and/or an impaired responsiveness to antiplatelet therapy are associated with the occurrence of stent thrombosis is growing, and this opens doors to a new era of tailoring the antiplatelet therapy itself. Although the most predictive and easy-to-use platelet function assay has not yet been identified, ongoing research will elucidate which aspects of platelet function are most involved in the pathogenesis of stent thrombosis.

