Implantable cardiac monitors (ICMs) detect atrial fibrillation (AF) in nearly 30% of patients within 30 days following patent foramen ovale (PFO) closure1, but the true incidence remains unknown because of limited preclosure AF screening in prior studies. The objective of this cohort study, involving patients who underwent continuous monitoring with an ICM before and after PFO closure, was to determine the true incidence and time-to-onset of postprocedural AF.
This prospective cohort study included consecutive patients who underwent PFO closure at Toulouse University Hospital between January 2015 and November 2022. Inclusion criteria were high-risk PFO (≥25 bubbles on contrast testing, atrial septal aneurysm with biphasic excursion >15 mm, or PFO width >2 mm) associated with cryptogenic embolism and preprocedural ICM monitoring; exclusion criteria were AF detected on ICMs prior to PFO closure and no postprocedural ICM monitoring.
The primary endpoint was the first AF episode lasting >30 seconds at 30 days and 1 year post-PFO closure. The cohort entry date was the day of PFO closure, with follow-up until the first event among the following: primary endpoint, death, last ICM update, 1 year, or the study’s conclusion (28 February 2023). The Kaplan-Meier method was used to estimate the cumulative AF incidence at 30 days and 1 year. Analyses were conducted using SAS software, v8.3 (SAS Institute).
According to French law on ethics, all patients received information about anonymised data collection, and the study was registered (registration number: RnIPH 2025-46) and covered by the MR-004 (CNIL number: 2206723 v 0).
A total of 126 patients (mean age 57.2±11.5 years, female sex 38.9%) were included. The most used PFO closure devices were the Amplatzer PFO Occluder ([Abbott] 76 patients, 60.3%) and the Amplatzer Cribriform Septal Occluder ([Abbott] 43 patients, 34.1%). The left disc size was ≤25 mm in most cases (106 patients, 84.1%). AF was excluded before PFO closure through ICM monitoring (median duration 8.6 months [interquartile range {IQR} 6.5-11.7]). Reveal devices (Medtronic) were the most frequently used ICMs (109 patients, 86.5%). During the first year post-PFO closure (median follow-up 5.8 months [IQR 3.3-11.3]), 32 patients (27.6%, 95% confidence interval [CI]: 20.1-37.1) experienced AF. At 30 days, 24 patients (19.3%, 95% CI: 13.4-27.4) experienced AF (Central illustration). The median time to the first AF episode was 20.5 days (IQR 15.5-44.0). Among 32 patients with AF, 5 (15.6%) were symptomatic, 5 (15.6%) required antiarrhythmic drugs, 25 (78.1%) received oral anticoagulation, and 2 (6.3%) had unplanned consultations. All AF episodes were paroxysmal, lasting <1 hour in 18 patients (56.3%).
This is the first cohort of patients to undergo PFO closure with extensive AF screening both before and after the procedure using ICMs, thus providing an accurate assessment of the true incidence of postprocedural AF. The monitoring duration before PFO closure of nearly 9 months ruled out pre-existing AF2. Nevertheless, the postprocedural AF incidence was high (19.3% at 30 days, 27.6% at 1 year), though lower than in previous ICM-based studies1, likely due to thorough AF screening prior to PFO closure.
Postprocedural AF mainly occurred within the first month, which is consistent with previous studies where approximately 80% of AF episodes occur during the first 30 days3. It has been hypothesised that early postprocedural AF is caused by local atrial stretch and irritation induced by the PFO closure device, and this is reinforced by the recent results of the AFLOAT trial3, where flecainide did not prevent AF after PFO closure.
Surprisingly, a significant proportion (25%) of AF episodes occurred beyond 30 days, even though pre-existing AF prior to PFO closure had been excluded. These late AF episodes therefore do not reflect undiagnosed pre-existing AF and might instead be induced by the PFO closure device. The underlying mechanism of these late AF episodes remains to be determined, and further studies are needed to assess their clinical burden and associated risk of thromboembolism.
Finally, despite being frequent, postprocedural AF was mostly asymptomatic and paroxysmal, consistent with the 3-5% incidence of clinical AF reported in clinical trials. These findings support the use of antiarrhythmic drugs only in selected and symptomatic patients.
This study has some limitations. The follow-up duration was limited, and our study population was older compared to those in clinical trials. This can be explained by the fact that we selected patients who underwent AF screening prior to PFO closure using ICMs, a strategy typically applied to those at higher risk of AF and who are, therefore, often older.
In this cohort of 126 patients with extensive AF screening both before and after PFO closure, the postprocedural AF incidence was high (19.3% at 30 days, 27.6% at 1 year), but it was mainly asymptomatic and paroxysmal. A significant proportion of the AF episodes occurred beyond 30 days, and management of these late AF episodes remains to be determined.
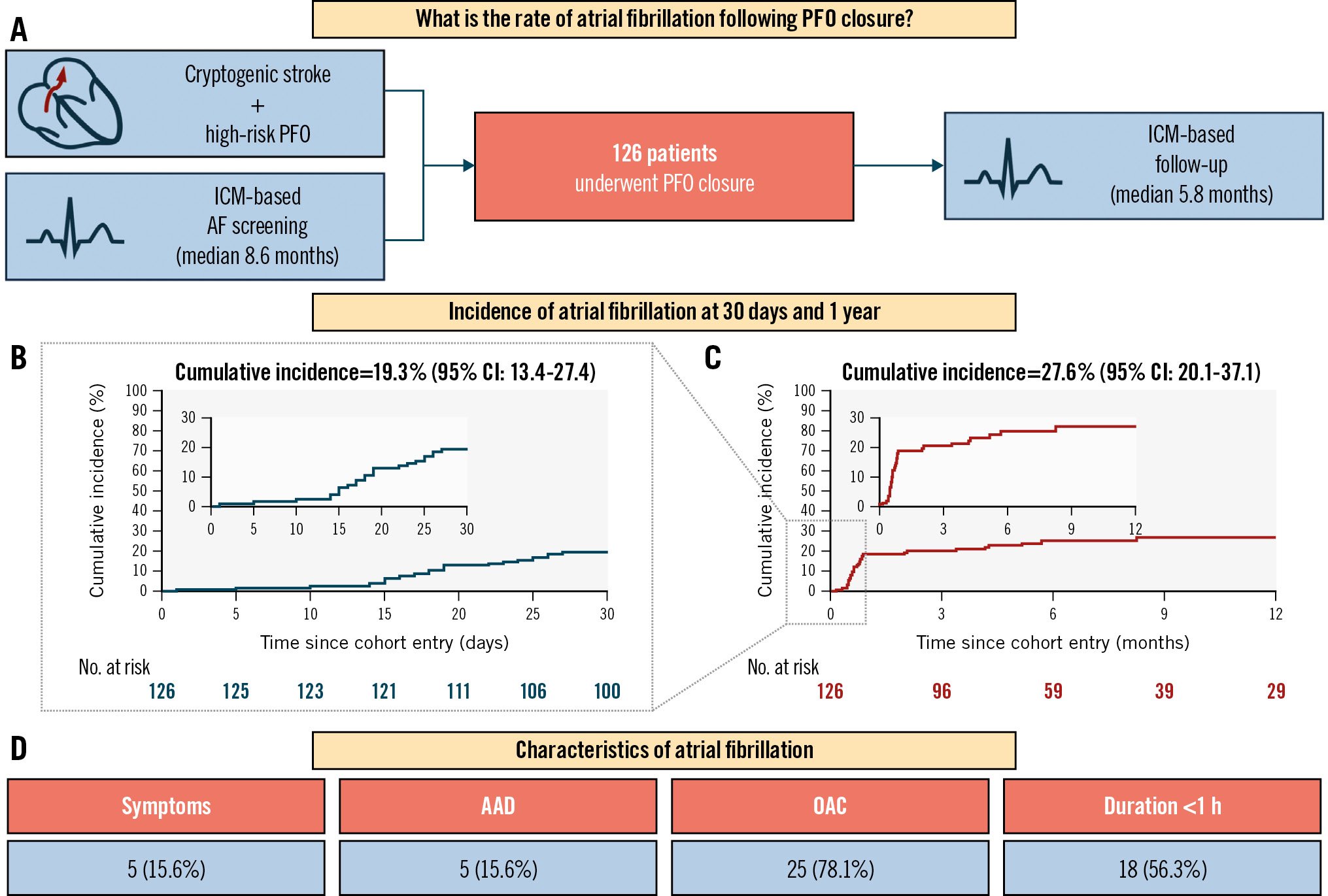
Central illustration. AF incidence following PFO closure. A) Study plan. Cumulative incidence of AF episodes lasting >30 seconds following PFO closure, assessed using the Kaplan-Meier method at 30 days (B) and 1 year (C). D) Distribution of AF characteristics. AAD: antiarrhythmic drugs; AF: atrial fibrillation; CI: confidence interval; ICM: implantable cardiac monitor; OAC: oral anticoagulation; PFO: patent foramen ovale
Conflict of interest statement
The authors have no conflicts of interest to declare regarding the submitted work.

