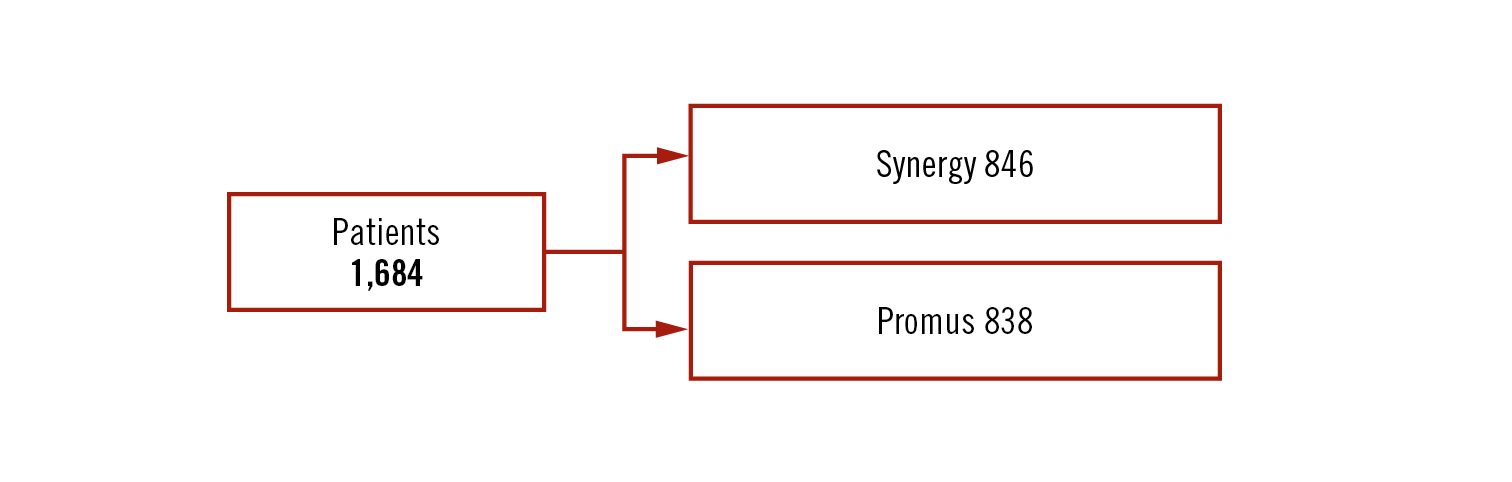
Objective
to report the 5-year clinical adverse event rate of the Synergy stent (thin-strut, bioresorbable polymer-coated everolimus-eluting stents) compared to Promus Element Plus stent
Study
multicentre, randomised, non-inferiority trial (margin 4.4%)
Population
patients with stable angina or non STEMI
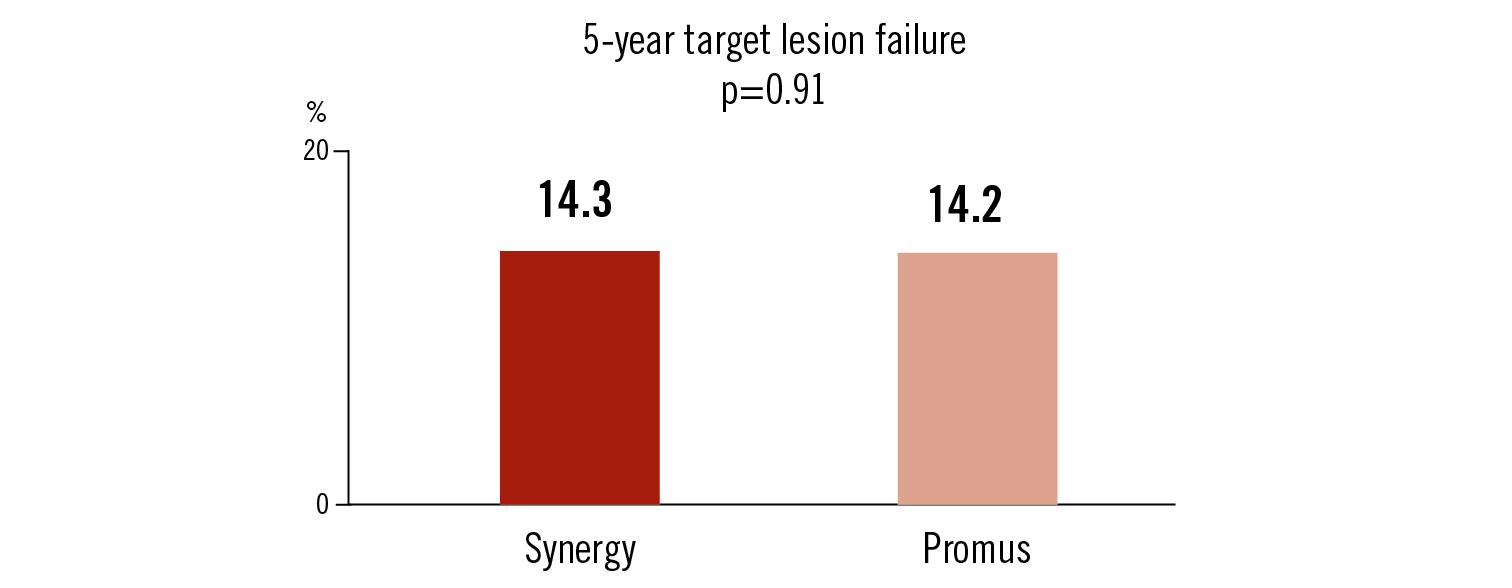
Endpoints
5-year target lesion failure: cardiac death, MI or ischaemia driven TLR
Conclusion
Synergy stent implantation was non-inferior compared to Promus stent implantation with respect to 5-year major adverse event rate in patients with stable CAD or non STEMI patients
Kereiakes et al. Circulation Cardiovasc Interv. 2019;12:e008152



