Objective
to report the 5-year clinical outcomes of BP-SES (biodegradable polymer sirolimus-eluting) as compared to DP-EES (durable polymer everolimus-eluting)
Study
prospective, international multicentre 2:1 randomised study (non-inferiority)
Population
patients with stable and acute syndromes (except STEMI)
Endpoints
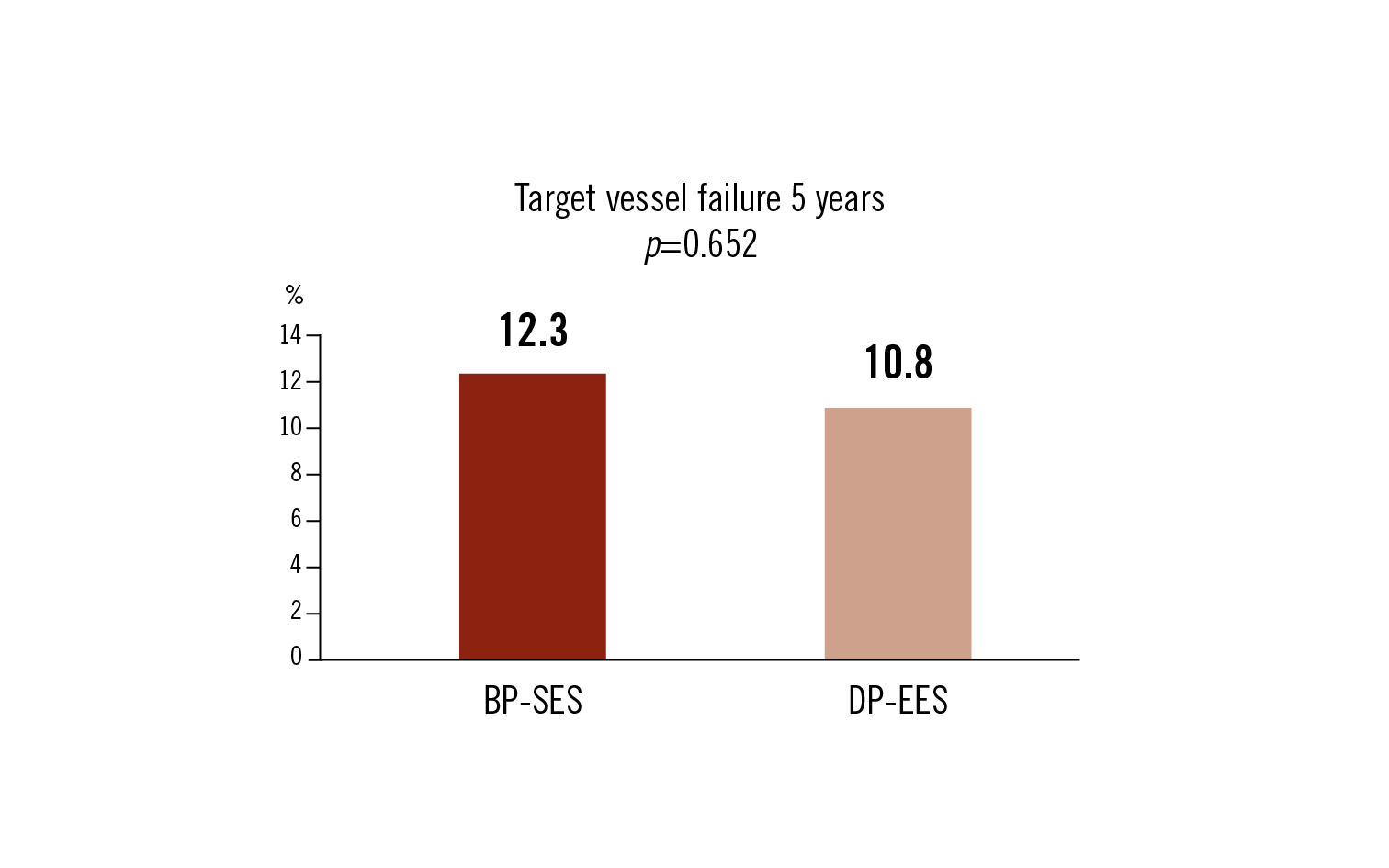
target vessel failure: cardiovascular death, target vessel MI and ischemic-driven lesion revascularisation at 5 years
Conclusion
there was no statistically difference of the incidence of 5-year target-vessel failure of CAD treatment with BP-SES as compared to DP-EES stents
Slagboom et al. Eurointervention 22-00526



