Objective
to assess the safety and efficacy of the Portico re-sheathable transcatheter aortic valve system as compared to any other transcatheter heart valve system
Study
multicentre, non-inferiority randomised trial (margin 8.5% safety and 8.0% efficacy)
Population
severe aortic stenosis at high and extreme high risk
Endpoints
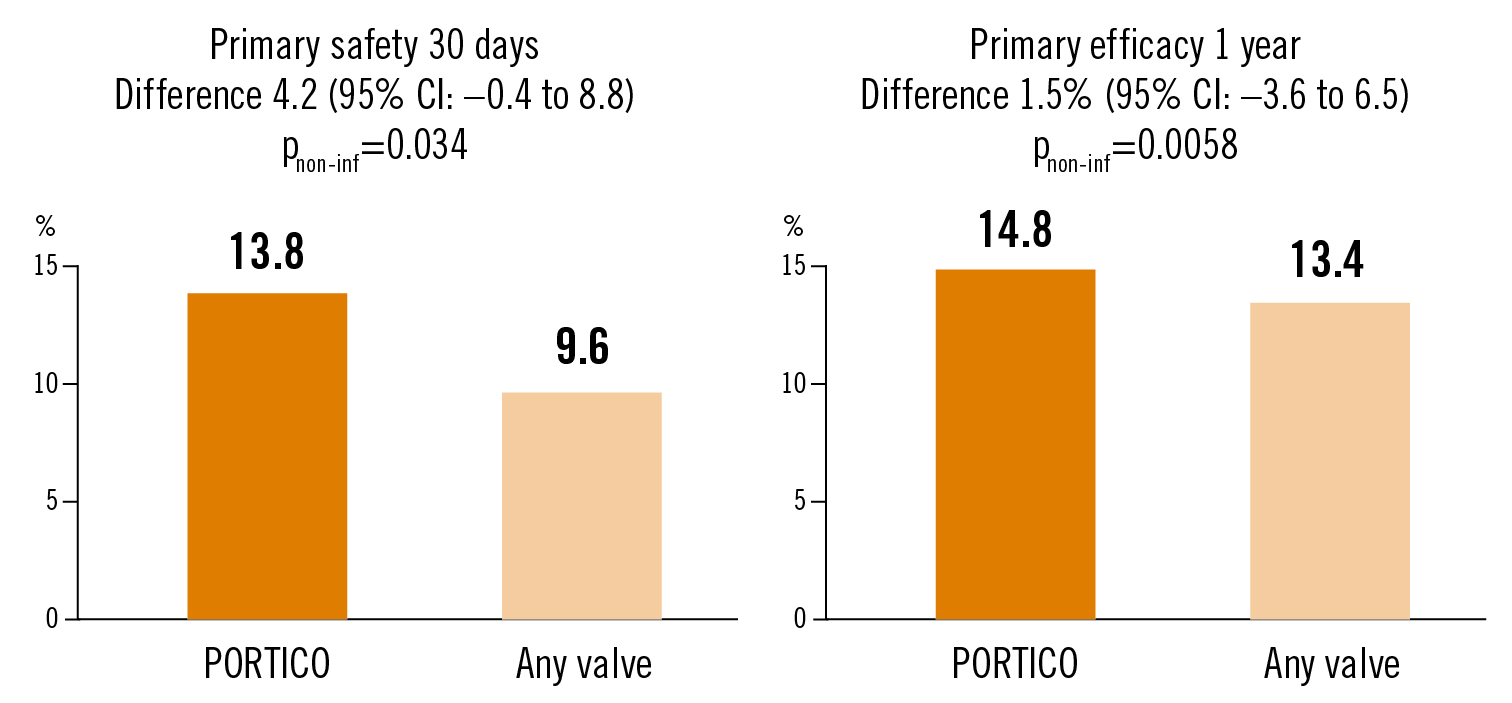
safety: all-cause death, disabling stroke, life-threatening bleeding, dialysis, major vascular complication at 30 days; efficacy: all-cause death, disabling stroke at 1 year
Conclusion
the PORTICO valve was associated with similar rates of death and disabling stroke at 1 year but MACCE (including death) was higher at 30 days compared to other commercial available valves
Makkar et al. Lancet. 2020;396:669-83



