Objective
to compare the efficacy of rivaroxaban (10 mg daily) with aspirin (75-100 mg daily for the first 3 months) as compared to aspirin (75-100 mg daily) with clopidogrel (at a dose of 75 mg daily during the first 3 months) in patients with successful TAVR
Study
open label, event-driven multicentre randomised study
Population
patients with successful TAVR and no established indication for long term anticoagulation
Endpoints
composite of all-cause death or thromboembolic events (stroke, MI, valve thrombosis, embolism, deep vein thrombosis or pulmonary embolism). Safety endpoint: composite of life threatening, disabling or major bleeding during median 17 months

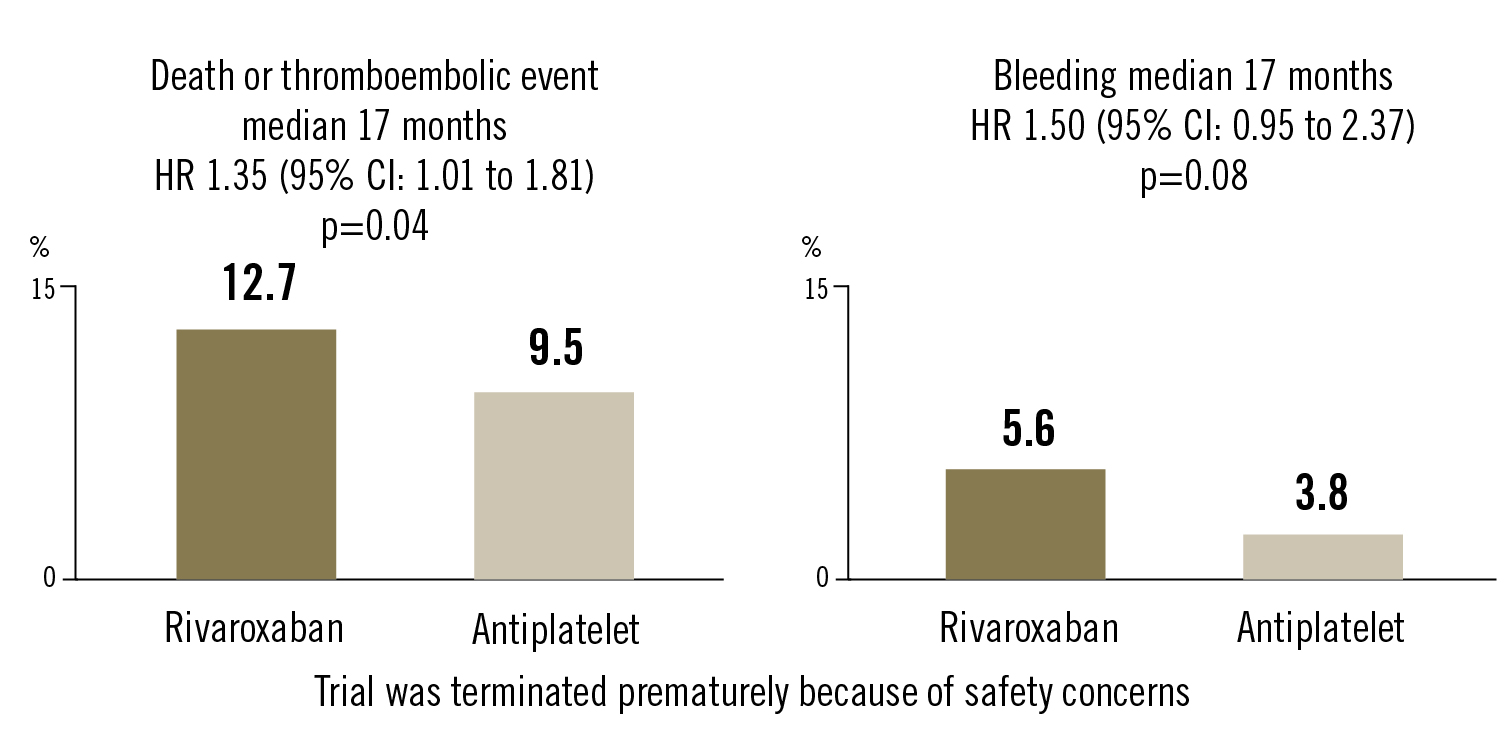
Conclusion
a treatment strategy of anticoagulation after TAVR including rivaroxaban (10 mg daily) was associated with higher adverse events and bleeding rates than antiplatelet based strategy
Dangas et al. N Eng J Med. 2020;382:120-29



