Objective
to report the 1-year adverse event rate of radial access compared to femoral access for treatment of CAD in patients with ACS
Study
multicentre, open-label superiority trial (two-nested trial)
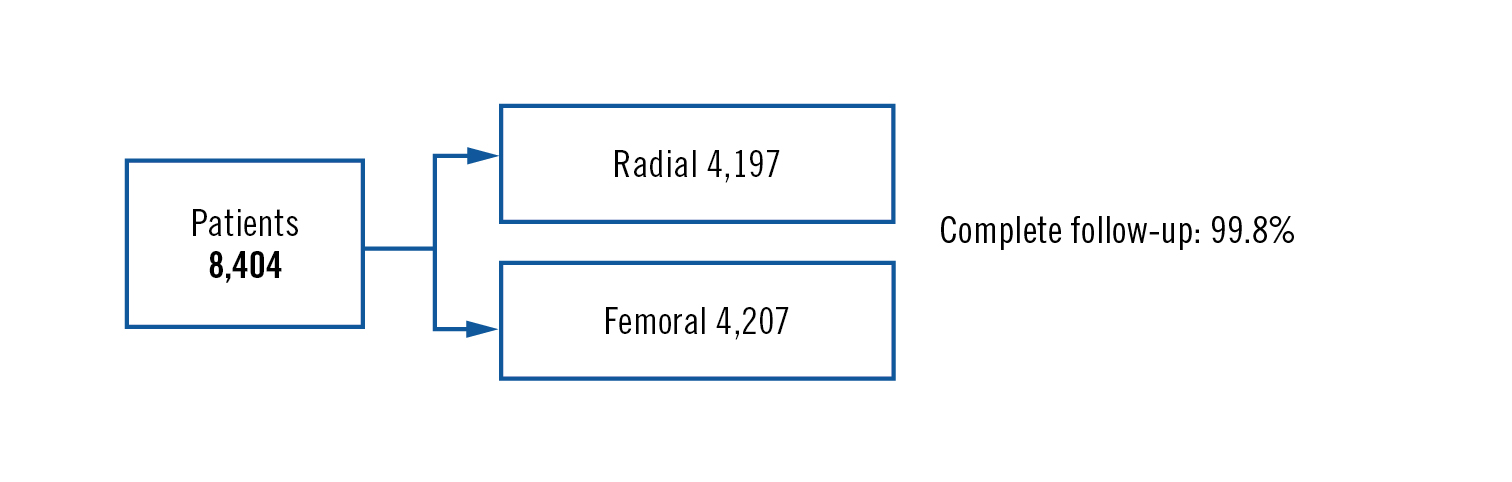
Population
patients with ACS
Endpoints
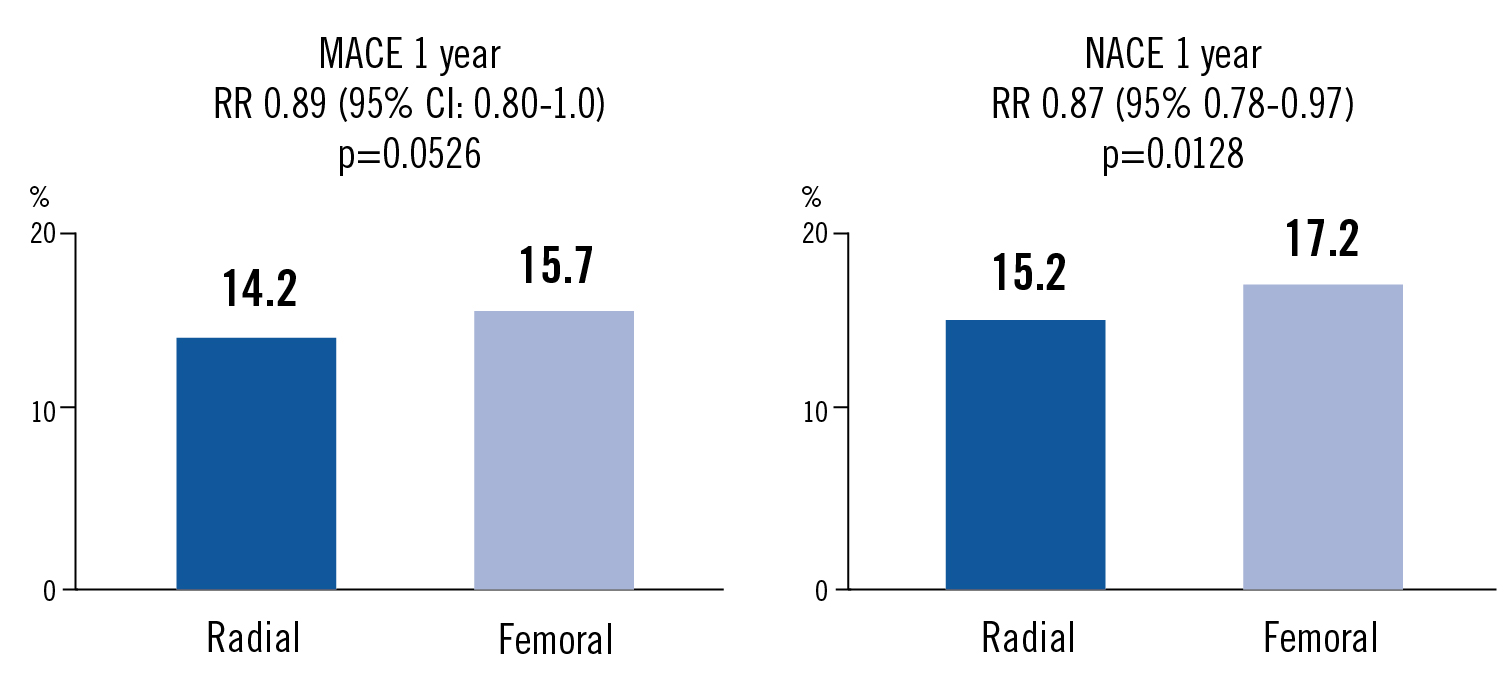
MACE as composite of all-cause death, MI or stroke at 1 year. NACE (net adverse clinical events): major bleeding or MACE
Conclusion
the major adverse 1-year event rate was not different between radial and femoral access, but the 1-year net adverse clinical event rate was lower with radial access compared to femoral access
Valgimigli et al. Lancet. 2018;392:835-48



