Objective
to assess the efficacy and safety of a dedicated plug-based vascular closure device (VCD) compared to a suture-based VCD during TAVR
Study
2-centre pilot randomised trial
Population
patients undergoing TAVR, MANTA (plug based VCD) and 2 Pro Glides (suture-based VCD) were used
Endpoints
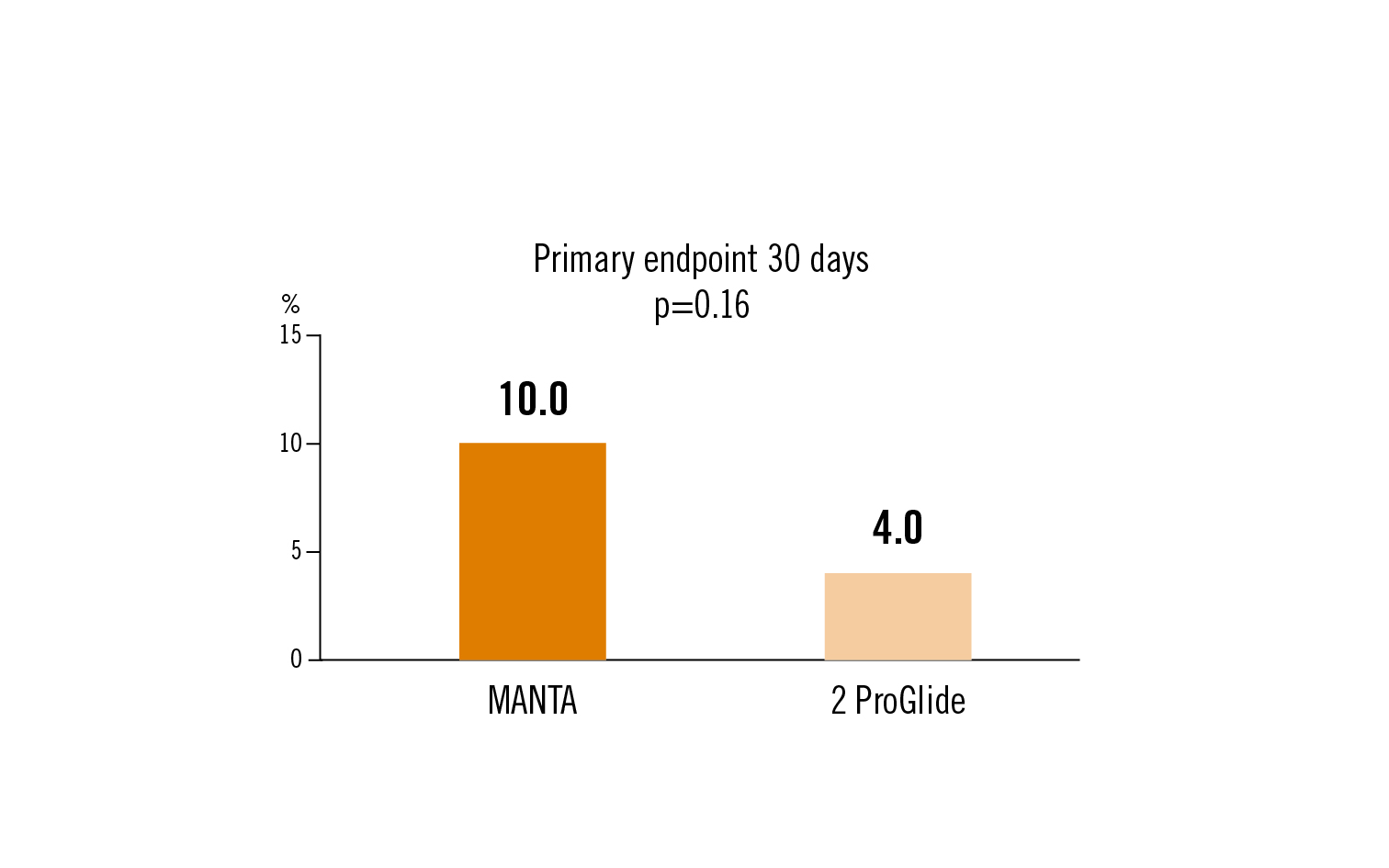
access site-related major or minor vascular complications (VARC-2) at 30 days

Conclusion
plug-based vascular closure device was not superior to suture-based closure during TAVR in terms of VARC-2 vascular complications
van Wiechen et al. J Am Coll Cardiol Interv. 2021;14:149-57



