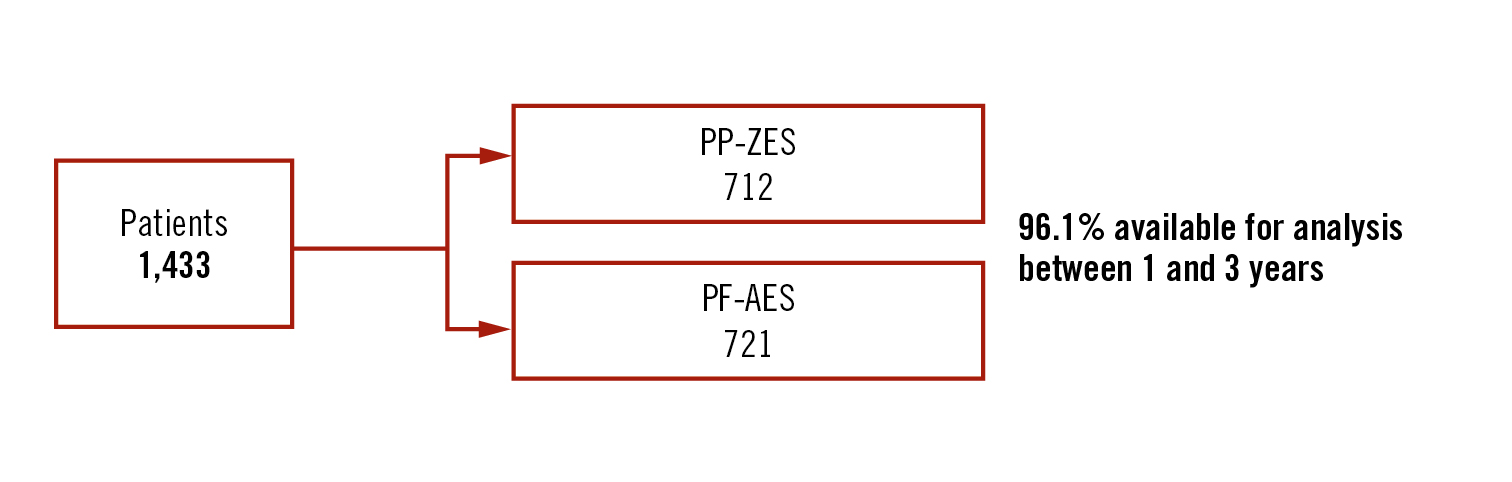
Objective
to assess the 3-year clinical outcomes of the polymer-free amphilimus-eluting stent (PF-RES) compared with permanent polymer zotarolimus-eluting stent PP-ZES
Study
physician-initiated multicentre non-inferiority randomised trial (margin 3.5%)
Population
all-comers
Endpoints
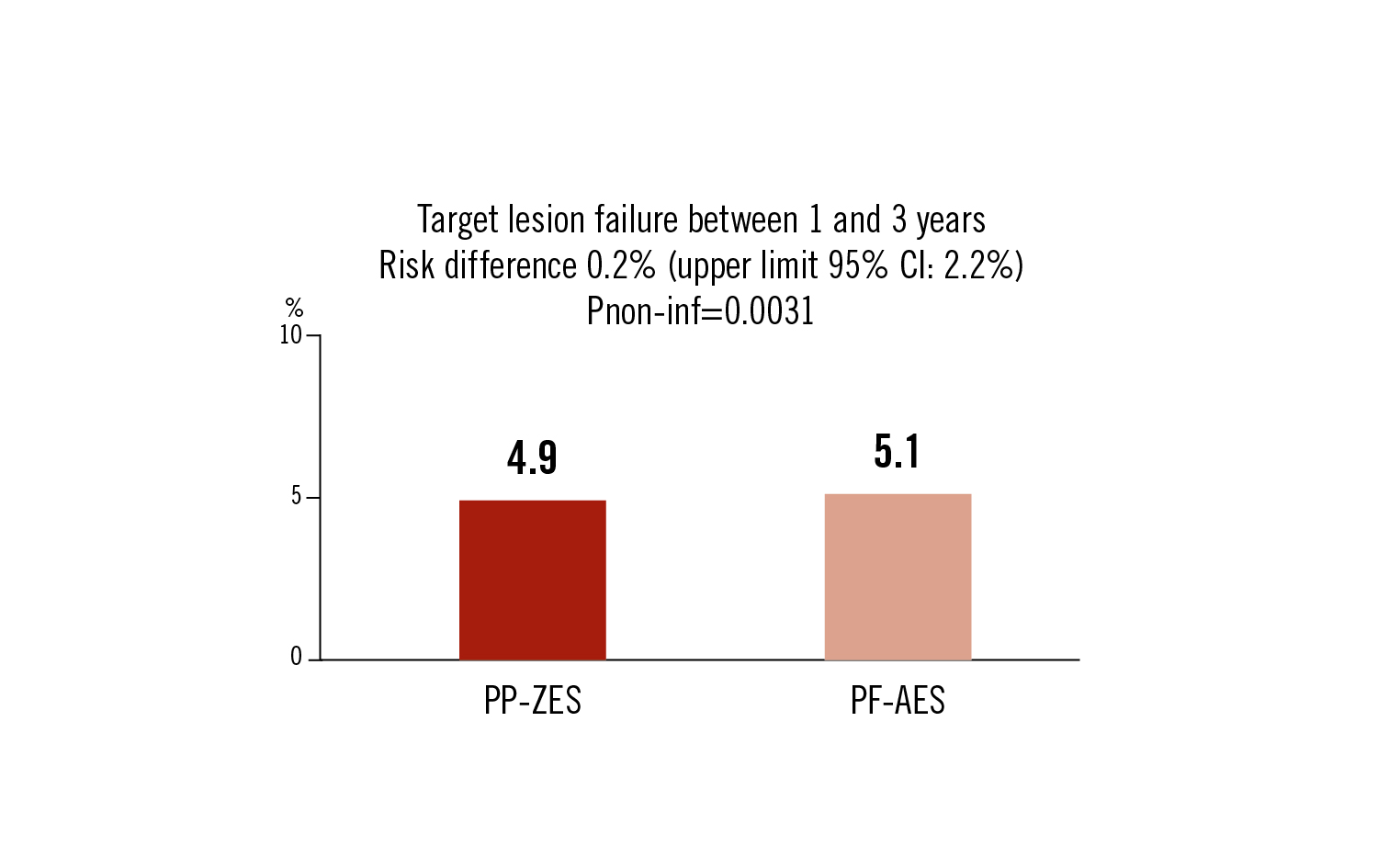
target lesion failure as a composite of cardiac death, MI, and target lesion revascularisation between 1 and 3 years follow-up
Conclusion
PF-AES stents are clinically non-inferior to PP-ZES DES in all-comers with respect to clinically adverse events between 1 and 3 years after implantation
van Hemert et al. J Am Coll Cardiol Intv. 2021;14:2477-86



