Objective
to compare mortality rates in patients treated with paclitaxel-coated versus uncoated devices undergoing peripheral arterial intervention
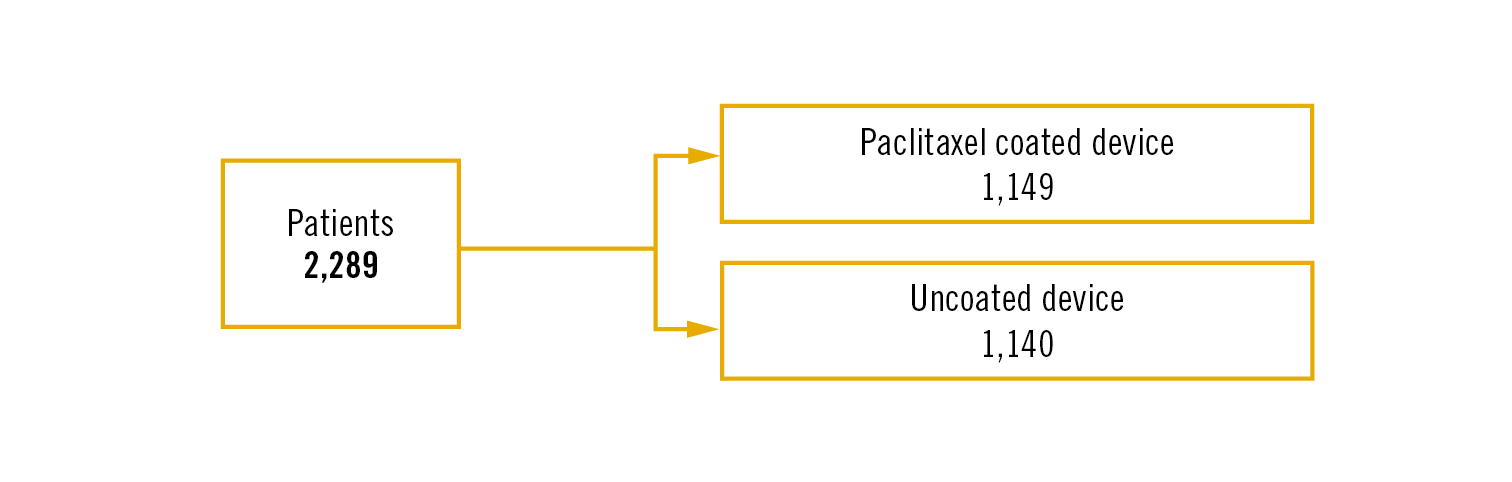
Study
multicentre, randomised, open-label, registry-based clinical trial
Population
patients with chronic limb ischaemia or intermittent claudication and stenosis >50% in infrainguinal arteries
Endpoints
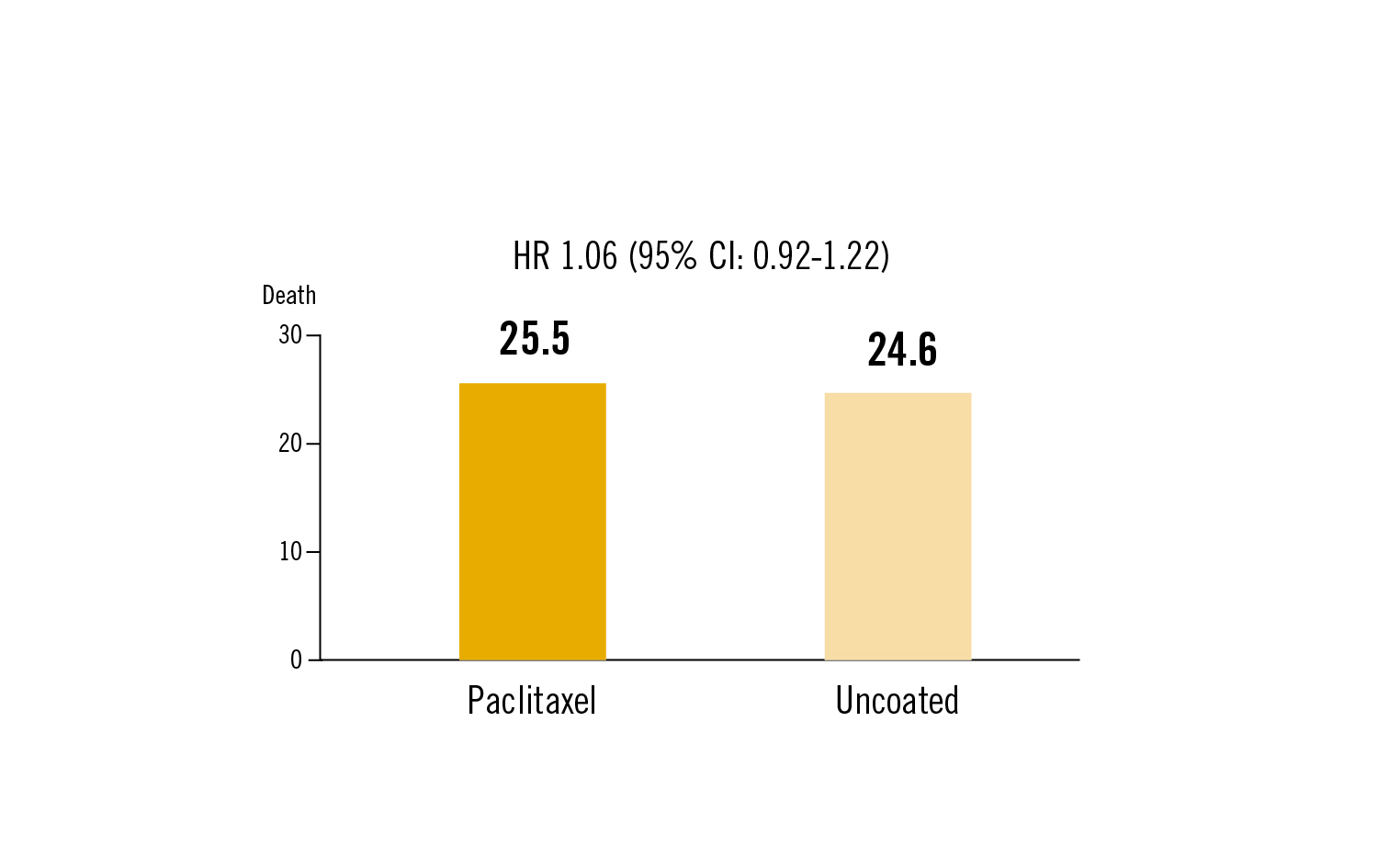
single end point for this interim analysis was all-cause mortality at mean follow-up of 2.49 years
Conclusion
in patients undergoing endovascular intervention or symptomatic peripheral arterial disease no difference in survival was seen with drug-coated versus uncoated devices at mid-term follow-up
Nordanstig et al. N Engl J Med. 2020;383:2538-46



