Objective
to report the 3-year clinical outcomes of MiStent biodegradable polymer crystalline sirolimus-eluting stent as compared to Xience permanent polymer everolimus-eluting stent
Study
multicentre, single-blinded randomised trial
Population
all-comers
Endpoints
composite cardiac death, target vessel MI and clinically indicated target lesion revascularisation at 3 years

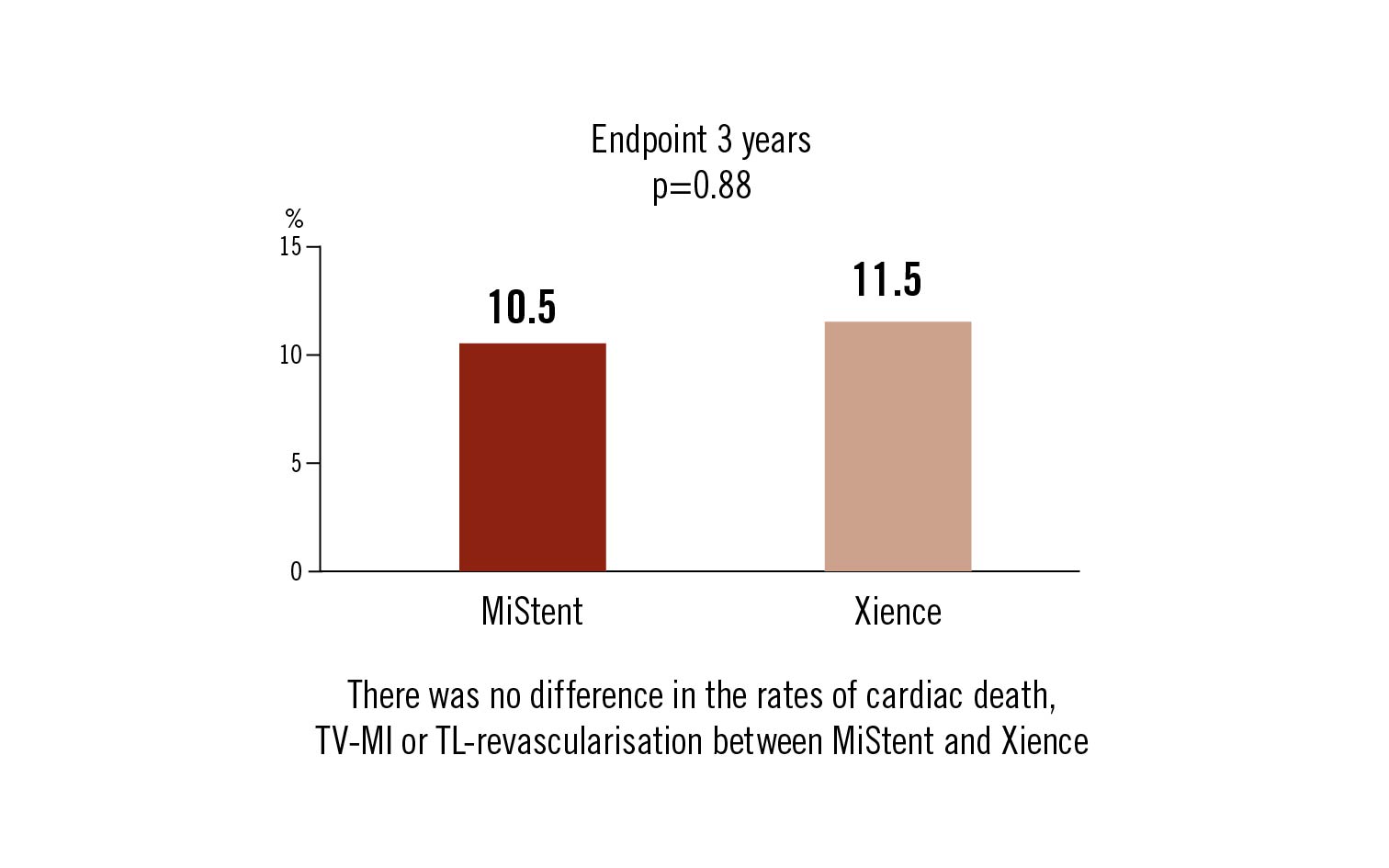
Conclusion
the major adverse event rate at 3 years was not statistically different between MiStent and Xience stent implantation in all-comers
Takahashi et al. Circulation Cardiovascular Intervention. 2020 (June);13:e008737



