Objective
to describe the efficacy and safety of renal artery denervation at 36 months
Study
prospective, single-blind, multicentre, sham-controlled, randomised clinical trial
Population
adults 18-80 with treatment resistant hypertension on stable, maximally tolerated doses of three or more antihypertensives, including a diuretic. Seated office BP >160 mmHg systolic and 24 hr ambulatory BP >135 mmHg systolic
Endpoints
changes in systolic blood pressure between the renal artery denervation group and the sham control groups at 36 months
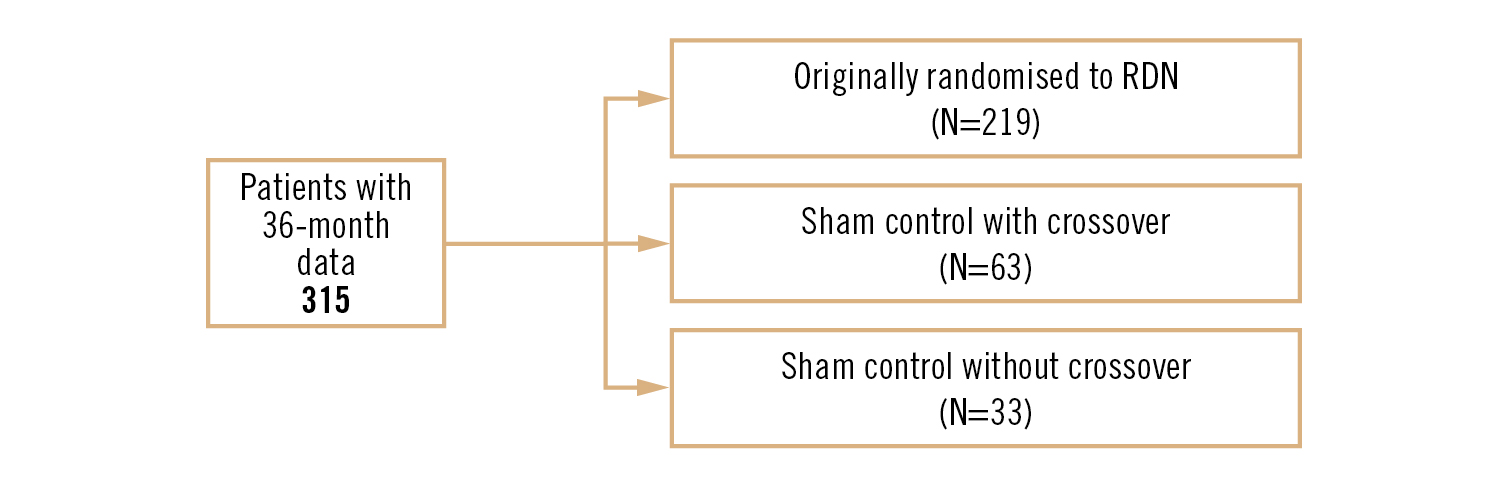
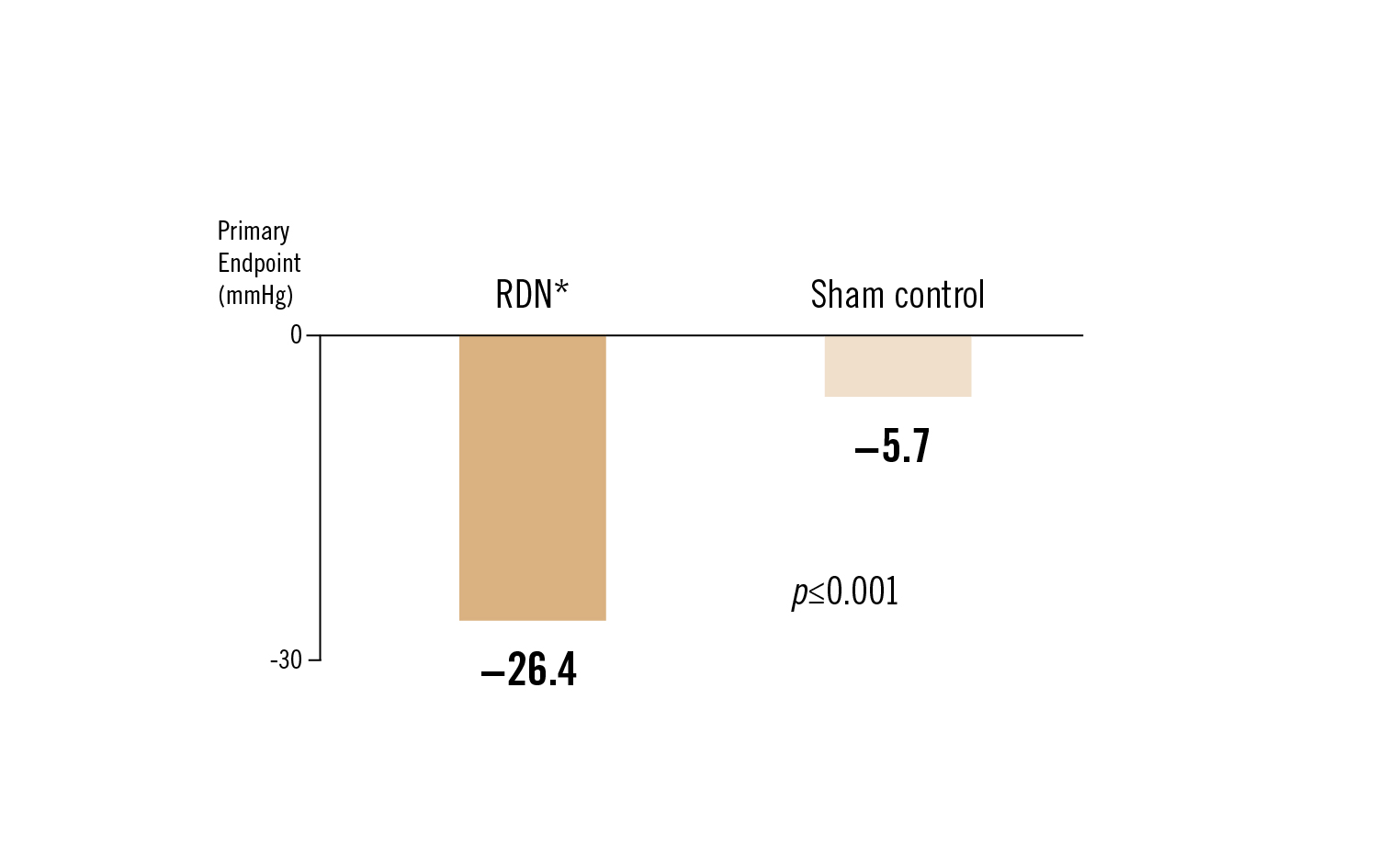
Conclusion
those patients treated with RDN, whether by initial randomisation or by crossover after unblinding at 6 months, were seen to have significant reductions in both office and 24-hour ambulatory blood pressure at 36 months, with no signal of late-emerging complications from RDN
Bhatt et al Lancet 2022; 400 (10361), 1405â16



