Objective
to determine whether CYP2C19 genotype guided antiplatelet therapy for selection or oral P2Y12 inhibitors does reduce adverse events during PPCI
Study
open-label, assessor-blinded randomised trial (non-inferiority )
Population
patients undergoing PPCI. In genotype guided arm carriers of loss-of function alleles (CYP2C19 *2 or *3) received ticagrelor or prasugrel, non-carriers received clopidogrel. Standard treatment : prasugrel or ticagrelor
Endpoints
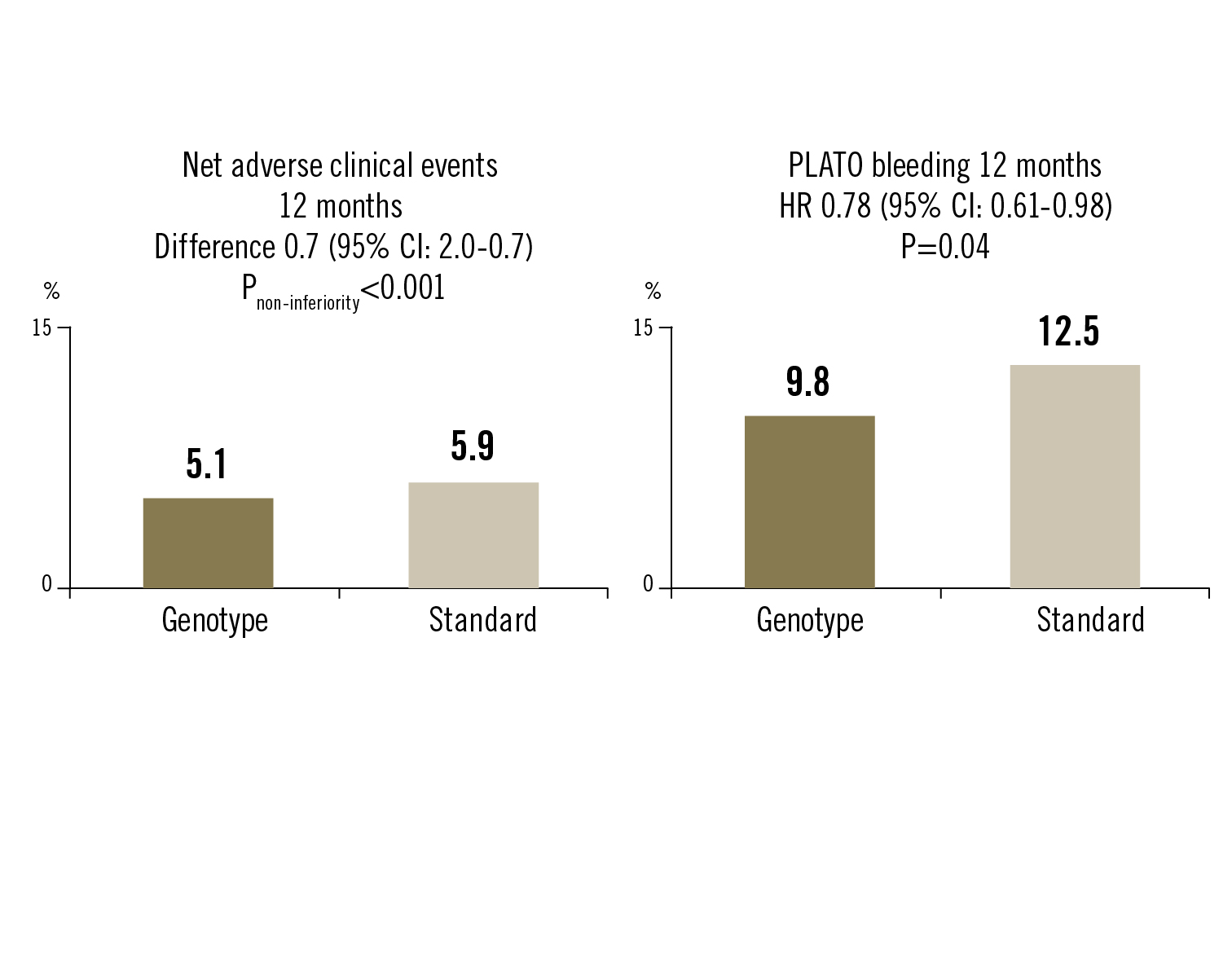
net adverse clinical events: all-cause death, MI, Stent thrombosis, stroke or major bleeding (PLATO criteria) at 12 months or PLATO major/minor bleeding at 12 months
Conclusion
CYP2C19 genotype guided strategy for selection of oral P2Y12 inhibitor was non-inferior to standard treatment at 12 months and was associated with a lower bleeding incidence in patients undergoing primary PCI
Claassens et al. N Engl J Med. 2019;381:1621-31



