Objective
to compare the efficacy and safety of durable-polymer DES (DP-DES) with biodegradable DES (BP-DES) in patients with ACS
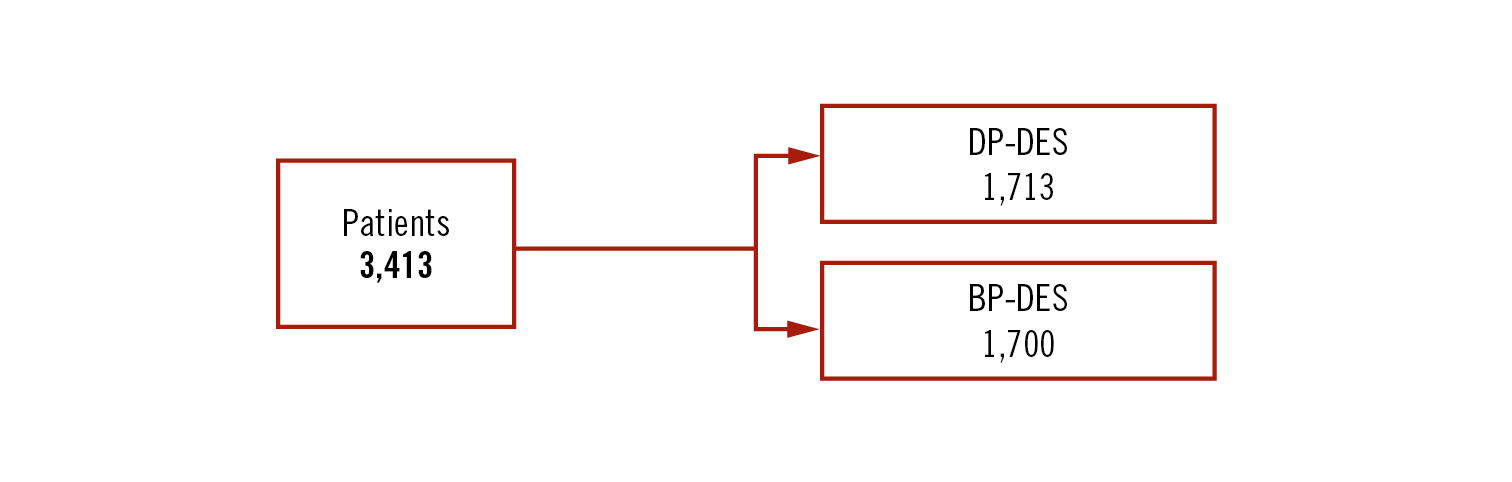
Study
investigator-initiated 2Ã2 factorial, multicentre randomised non-inferiority trial (margin 2.0%). The DES arm.
Population
patients with ACS
Endpoints
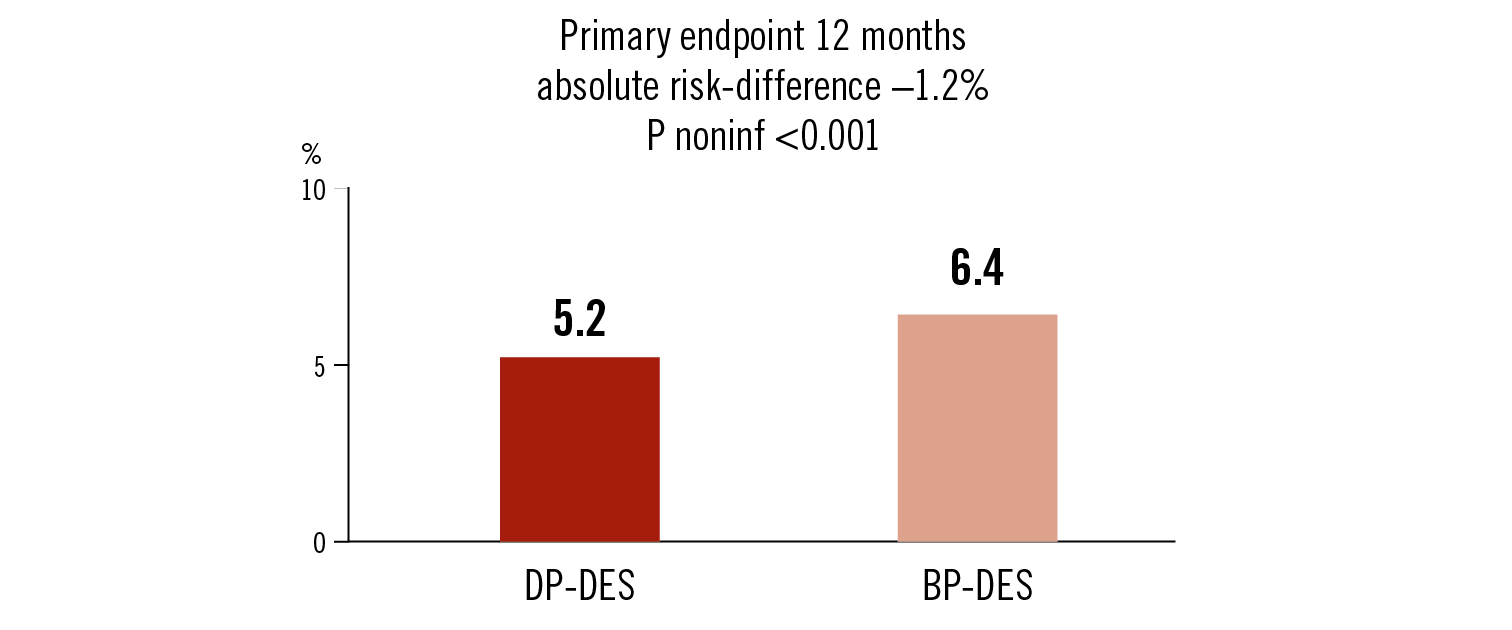
composite of all-cause death, non-fatal MI, or repeat revascularisation at 12 months
Conclusion
DP-DES was non-inferior to BP-DES at 12 months follow-up in patients with ACS undergoing PCI
Kim et al. Circulation. 2021;143:1081-91



