Objective
To measure the efficacy of Coronary Sinus Reducer (CSR) implantation compared to placebo on myocardial ischaemia reduction and symptom improvement.
Study
Investigator-initiated, double-blind, randomised, placebo-controlled trial.
Population
Patients with angina, epicardial coronary artery disease and ischaemia with no further options for antianginal therapy.
Endpoints
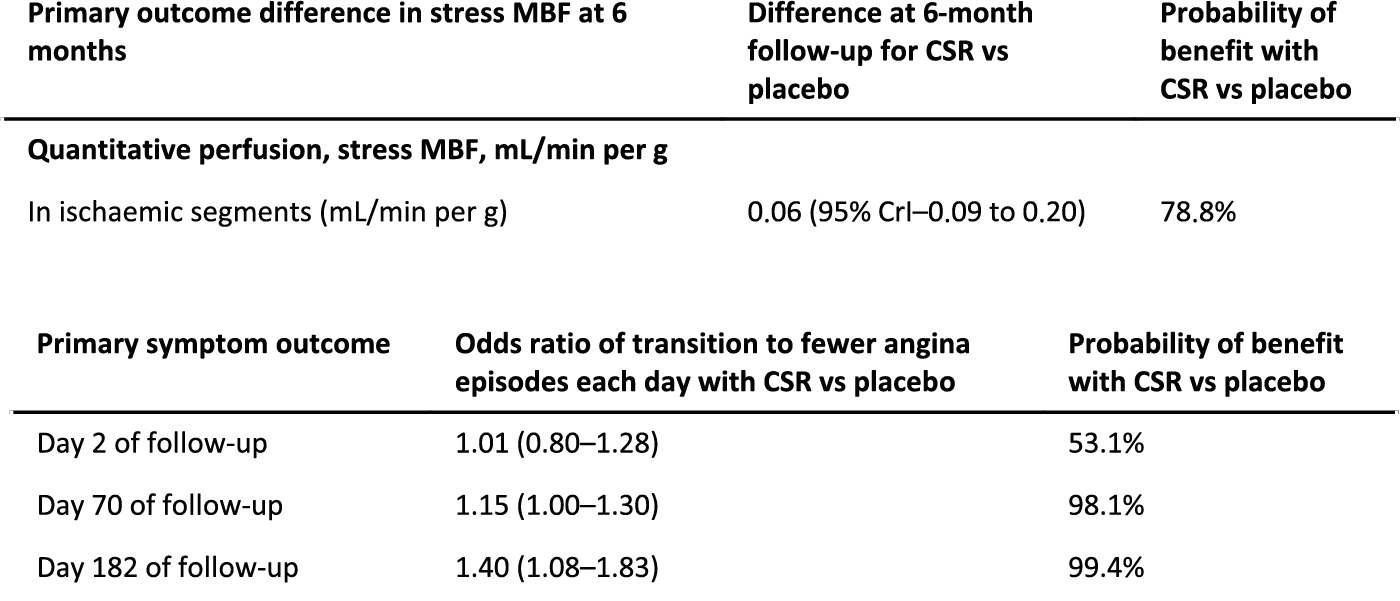
Co-primary outcomes. Myocardial blood flow (MBF) in ischaemic segments during adenosine-stress perfusion cardiac magnetic resonance scan. Number of daily angina episodes. At 6 months follow up.
Conclusion
Compared to placebo, an improvement in stress blood flow in ischaemic segments in patients with angina was not observed with CSR implantation though an improvement in number of daily angina episodes was observed.
Foley et al. Lancet 2024;20:216-223



