Objective
to compare the efficacy and safety of biodegradable polymer based sirolimus-eluting stents (BP-SES, YOKON CHOICE) vs. permanent polymer-based everolimus-eluting stents (PP-EES, XIENCE) vs. early generation permanent polymer-based sirolimus-eluting stents (PP-SES, CYPHER) during 10-year follow-up
Study
2 centre, prospective randomised trial
Population
â patients with ischemic symptoms and â¥50% de novo stenosis
â excluded: left main and cardiogenic shock
Endpoints
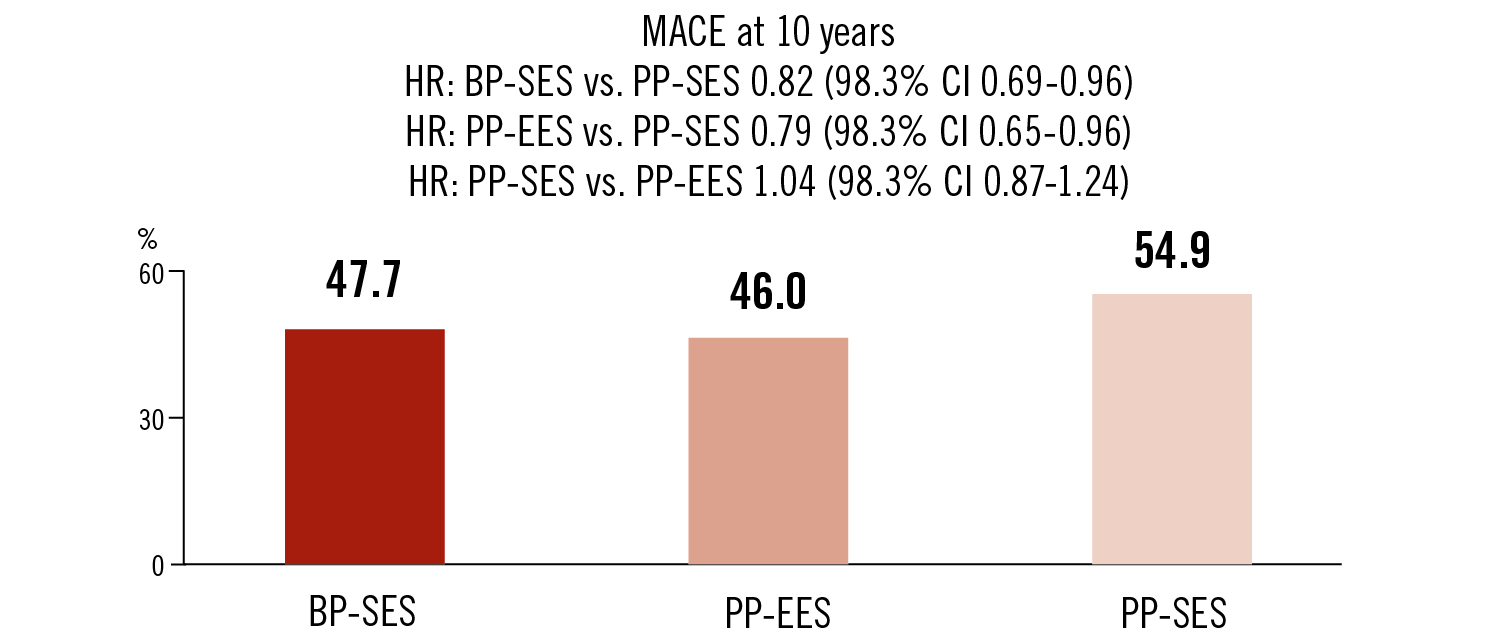
MACE as death, MI or target lesion revascularisation at 10 years
Conclusion
BP-SES and PP-EES showed comparable clinical outcomes at 10 years. PP-SES was associated with higher adverse event rate at 10 years
Kufner et al. Circulation. 2019;139:325-33



