Objective
to compare the safety and efficacy of a new biodegradable polymer sirolimus-eluting stent (ultimaster stent) BP-SES with a bare-metal stent (BMS) for the treatment of STEMI
Study
prospective single blind multicentre non-inferiority randomised trial (3:1) (margin 3%)
Population
patients undergoing primary PCI for STEMI
Endpoints
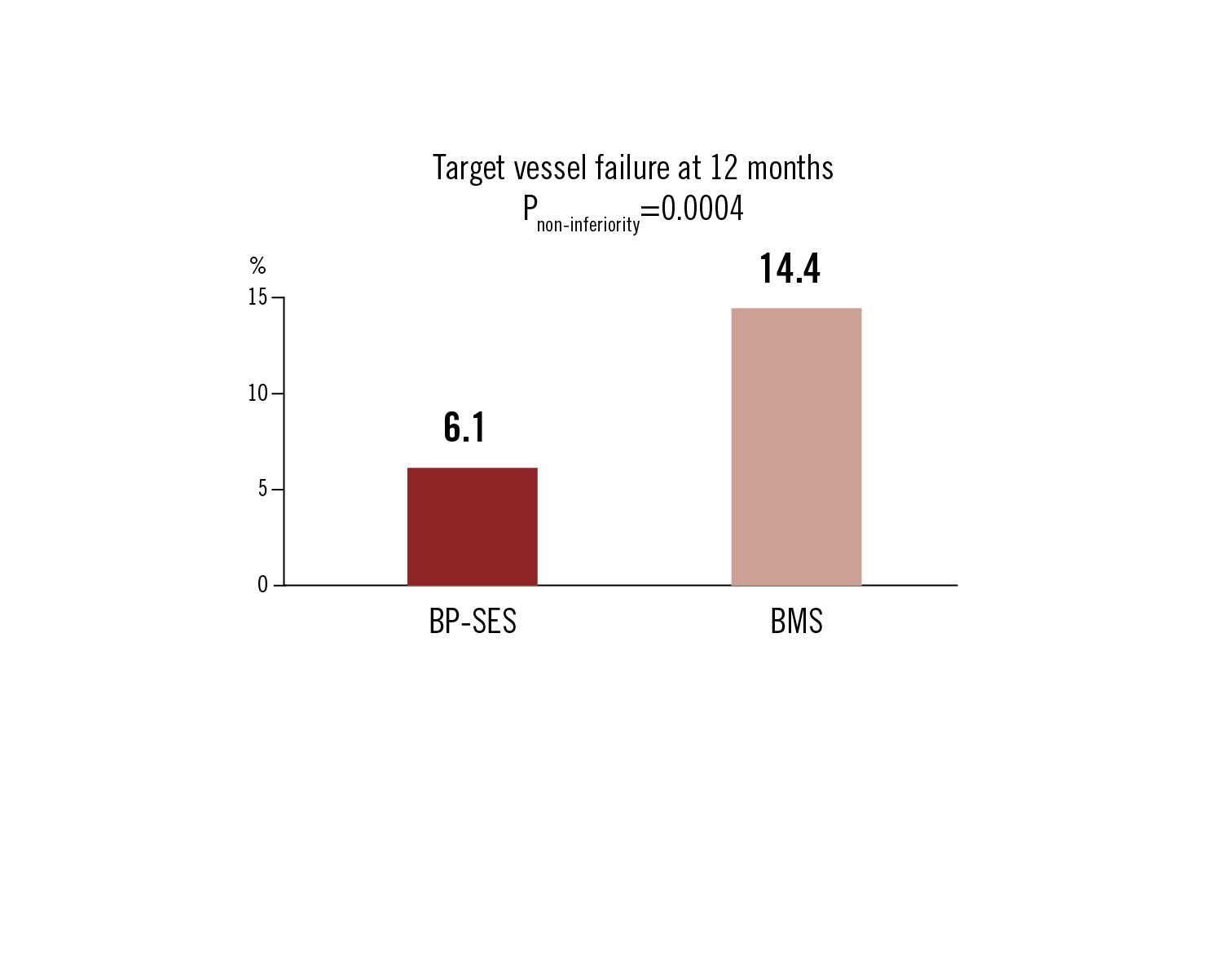
TVF defined as cardiac death, MI or clinically driven TVR at 12 months
Conclusion
BP-SES was clinically non-inferior to BMS for PCI treatment of STEMI
Valdes-Chavarri et al. EuroIntervention. 2019;14:e1836-42



