Objective
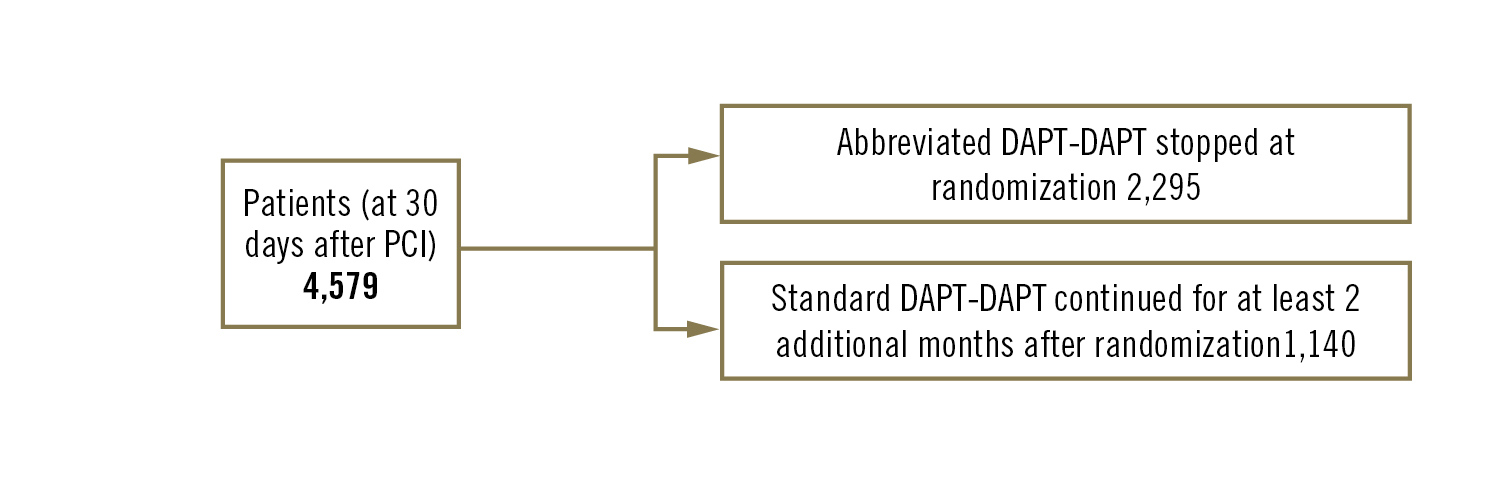
to compare rates of adverse clinical events in high bleeding risk patients randomised at 30 days after PCI to abbreviated vs standard dual antiplatelet therapy (DAPT)
Study
multicentre, randomised, open-label clinical trial
Population
coronary PCI patients with high bleeding risk
Endpoints
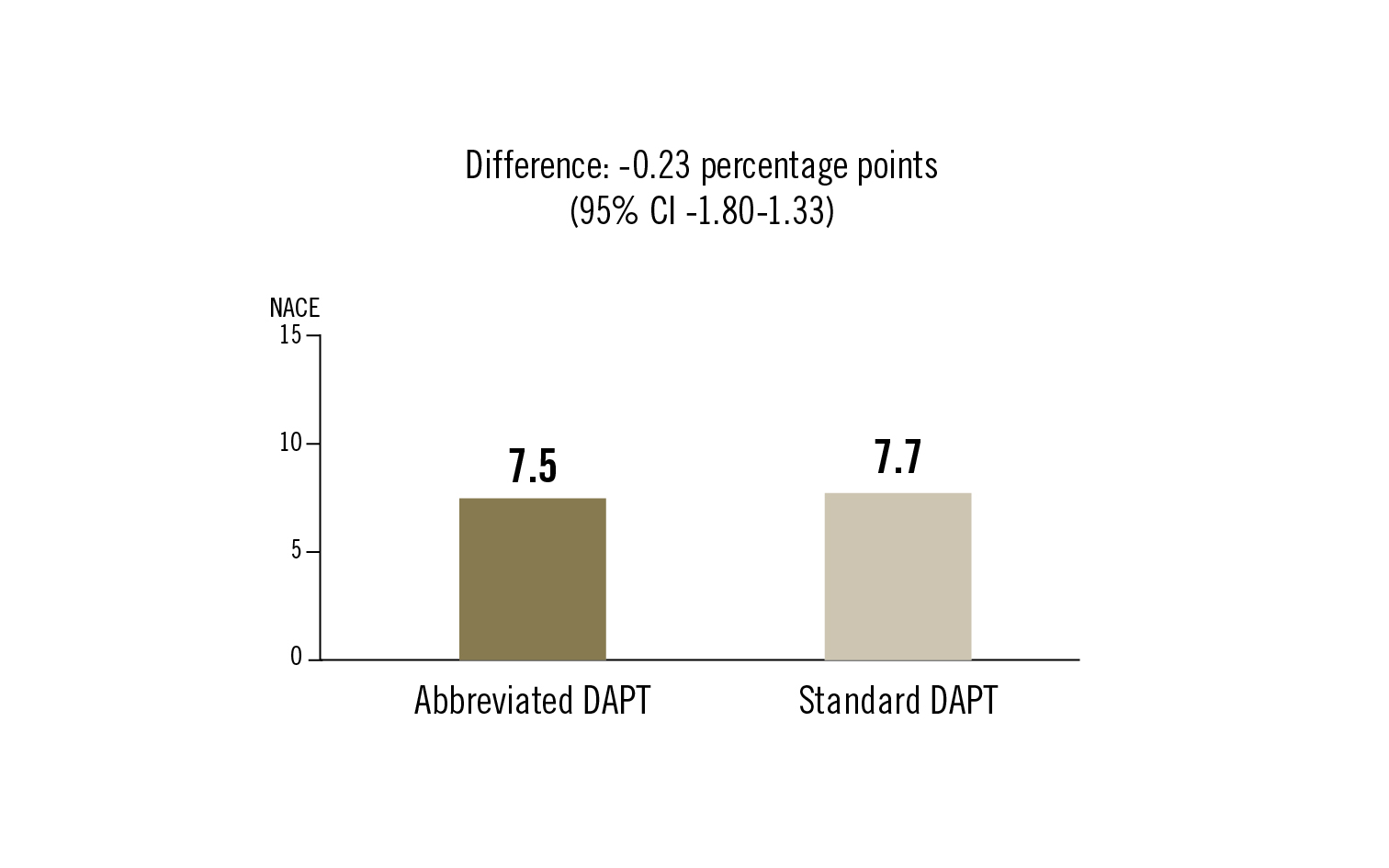
NACE (composite of all cause death, MI, stroke or major bleeding), MACCE (composite all cause death, MI, or stroke), and major or clinically relevant nonmajor bleeding; cumulative incidences assessed at 335 days
Conclusion
in high bleeding risk patients undergoing PCI one-month DAPT was noninferior to DAPT continuation for at least 2 additional months with regard to net adverse clinical events and major adverse cardiac or cerebral events. Abbreviated DAPT also had lower incidence of major/clinically-relevant nonmajor bleeding
Valgimigli et al. N Engl J Med. 2021;385:1643-55



