Objective
The aim of this trial was to assess whether the use of ticagrelor monotherapy after 1 month of DAPT, compared with 12 months ticagrelor plus aspirin DAPT, could reduce the incidence of clinically relevant bleeding events without an accompanying increase in major adverse cardiovascular or cerebrovascular events (MACCE).
Study
Prospective, double blind, multicentre randomised trial
Population
Patients with an ACS who completed the IVUS-ACS study and who had no major ischaemic or bleeding events after 1-month treatment with DAPT
Endpoints
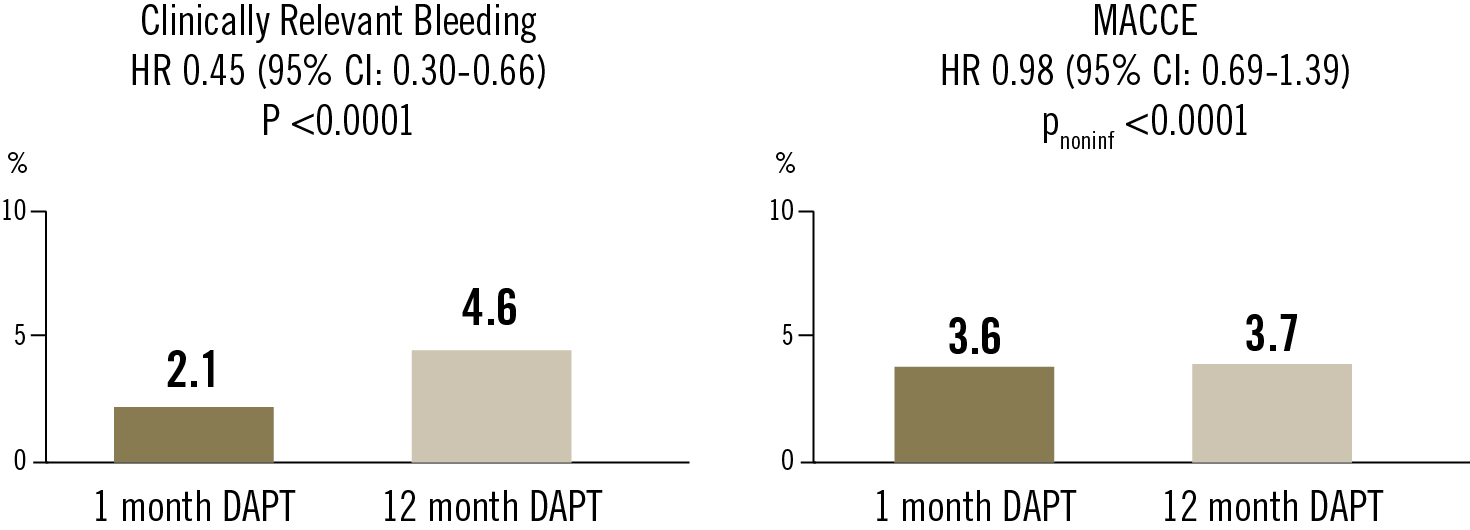
The primary superiority endpoint was clinically relevant bleeding (Bleeding Academic Research Consortium types 2, 3, or 5). The primary non-inferiority endpoint was MACCE (defined as the composite of cardiac death, myocardial infarction, ischaemic stroke, definite stent thrombosis, or clinically driven target vessel revascularisation)
Conclusion
In patients with ACS and stenting who remained event-free for 1 month on DAPT, treatment with ticagrelor alone between month 1 and month 12 after the intervention resulted in a lower rate of clinically relevant bleeding and a similar rate of MACCE compared with ticagrelor plus aspirin.
Ge et al. Lancet 2024



