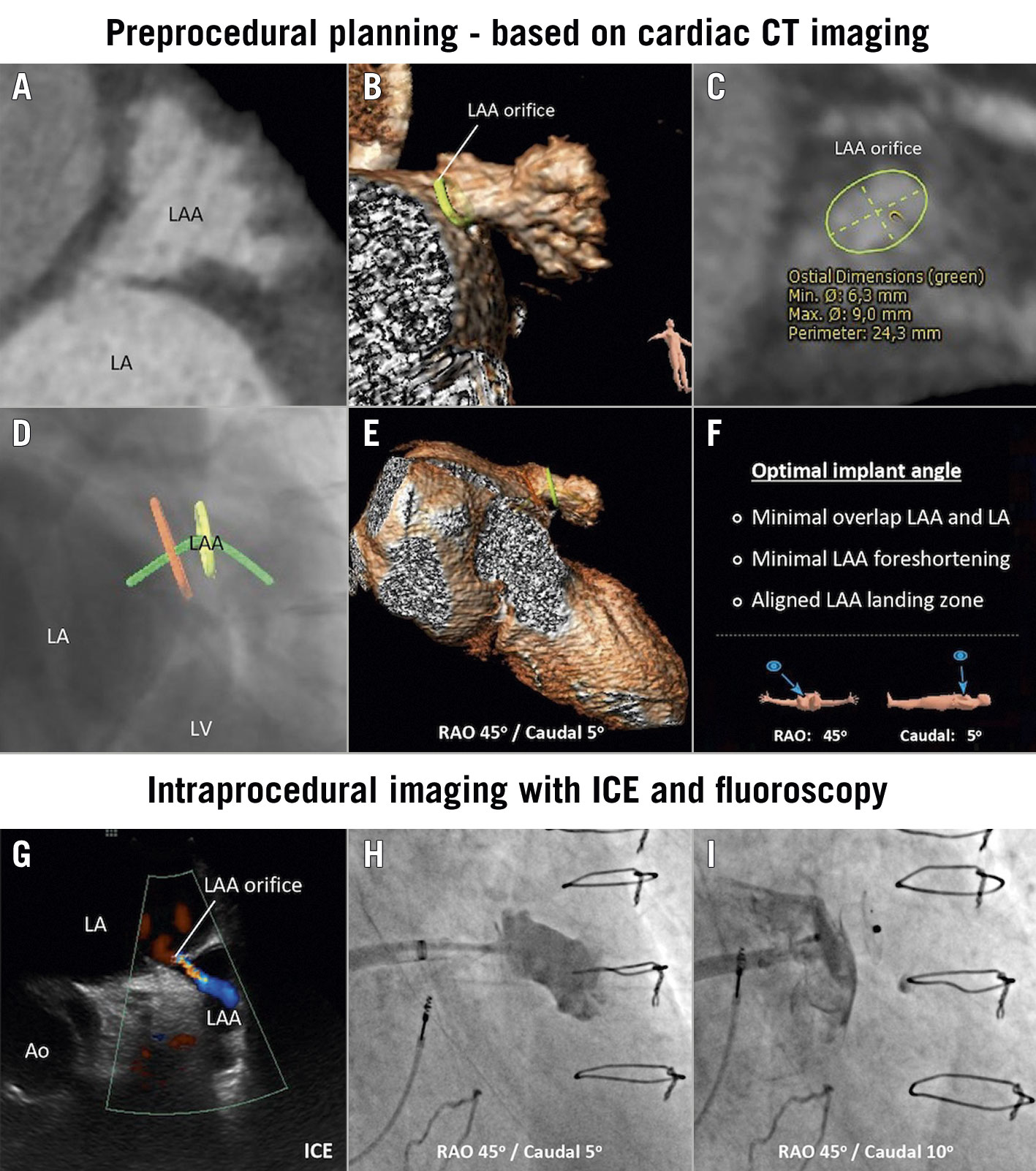
Figure 1. LAA closure in post-surgical ligation LAA. A) Post-surgical ligation LAA with small orifice, as shown at cardiac CT analysis. B) Three-dimensional reconstruction of the LAA. C) Cardiac CT measurements of the LAA orifice. D-F) Optimal predicted fluoroscopic projection or implant angle to facilitate cannulation of the small LAA orifice and secure a safe and effective LAA closure. G) ICE imaging from the LUPV position showing persistent flow into the LAA. H) Angiogram through 6 Fr multipurpose catheter confirming selective LAA cannulation. I) Final deployment of the AMPLATZER Vascular Plug III with successful LAA closure. Ao: ascending aorta; CT: computed tomography; ICE: intracardiac echocardiography; LA: left atrium; LAA: left atrial appendage; LUPV: left upper pulmonary vein; LV: left ventricle; RAO: right anterior oblique
A 56-year-old male, with permanent atrial fibrillation and a history of surgical mitral annuloplasty and ligation of the left atrial appendage (LAA), was referred for assessment due to recurrent ischaemic stroke despite direct oral anticoagulant (DOAC) therapy. The potential aetiologies for the recurrence of stroke include cardioembolic sources either from the residual LAA or the repaired mitral valve.
Cardiac computed tomography (CT) imaging confirmed persistent contrast filling in the LAA, with the post-ligation LAA orifice measured as 6.3 × 9.9 mm (Figure 1A-Figure 1C). A percutaneous LAA closure (LAAC) with intracardiac echocardiography (ICE) guidance under local anaesthesia was scheduled. One of the main challenges when having to cannulate such a small LAA orifice and secure a safe and effective LAA closure is to find an optimal fluoroscopic projection, in which there is (1) minimal overlap between the LAA and left atrium (LA), (2) minimal foreshortening of the LAA, and (3) an aligned LAA orifice and landing zone. This optimal implant angle could be determined before the procedure with cardiac CT analysis – i.e., a right anterior oblique (RAO) 45°/caudal 5° fluoroscopic view for this particular case (Figure 1D-Figure 1F).
Transseptal puncture was performed under ICE guidance, targeting the inferoposterior region of the fossa ovalis – as predicted by preprocedural cardiac CT analysis – to achieve the best trajectory for cannulation of the LAA orifice. An 8.5 Fr Agilis NxT steerable introducer (Abbott) was introduced into the LA over a 0.035” stiff guidewire, which was placed in the left upper pulmonary vein. The ICE probe was also advanced to the LA through the same transseptal puncture site for further intraprocedural guidance, confirming there was persistent flow into the LAA (Figure 1G, Moving image 1). Cannulation of the LAA with a standard pigtail catheter was, as anticipated, not feasible. Hence, a 6 Fr multi-purpose (MP) catheter and a 0.018” GLIDEWIRE ADVANTAGE guidewire (Terumo Corp.) were introduced through the steerable introducer. The LAA was successfully wired with the help of the steerable introducer. The contrast angiogram from the MP catheter confirmed the location of the catheter inside the LAA (Figure 1H, Moving image 2).
Closure with standard LAAC devices was considered but assessed as not optimal given the small LAA orifice. A 0.035” Amplatz Super Stiff guidewire (Boston Scientific) was introduced into the LAA. The steerable introducer was then gently railed over the guidewire to cannulate the LAA. Next, an AMPLATZER Vascular Plug III (AVP III; Abbott) size 14 mm × 5 mm was inserted into the steerable introducer by simply cutting the loader and introducing the device through the haemostatic valve of the Agilis NxT. The first deployment attempt was performed with the central waist occluding the orifice; ICE revealed that there was persistent peri-device flow across the LAA. The second deployment attempt was performed placing the disc and waist on the LAA side and the remaining disc on the LA side (Figure 1I). ICE confirmed a stable device position and no peri-device leak (Moving image 3). The final angiogram after device deployment confirmed successful LAAC (Moving image 4). The patient was discharged the next day after the procedure.
The occurrence of unsuccessful surgical LAA closure has been reported to be 60%1. In this challenging scenario, however, meticulous preprocedural planning with cardiac CT, intraprocedural guidance with ICE and a highly steerable introducer and guidewire are excellent tools for percutaneous LAAC.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Persistent flow into the left atrial appendage as shown in intracardiac echocardiography positioned in left atrium.
Moving image 2. Contrast angiogram from the multipurpose catheter confirming the location of the catheter inside the left atrial appendage.
Moving image 3. Stable device position and no peri-device leak as shown in intracardiac echocardiography positioned in left atrium.
Moving image 4. Final angiogram after device deployment confirming successful left atrial appendage closure.

